equation de goldman
| Chapter 1 The Hodgkin–Huxley Equations |
|
ELECTROPHYSIOLOGIE: PRINCIPES ET TECHNIQUES
Les équations de Goldman-Hodgkin-Katz (GHK) reposent sur plusieurs hypothèses déjà utilisées dans le modèle de l'électrodiffusion : - La membrane est homogène |
|
Goldman-Hodgkin-Katz Equations
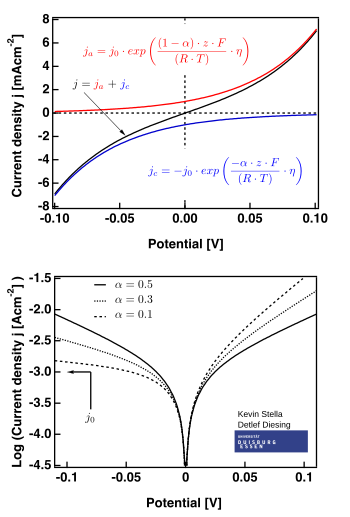
There are two Goldman-Hodgkin-Katz (GHK) equations: the GHK voltage equation and the GHK current equation The GHK voltage equation gives the membrane potential expected to result from multiple ion species at differing concentrations on either side of the membrane |
|
Lecture 1: Membrane Potential Nernst & Goldman Equations
A The equation that describes this is a modification of the Nernst equation called the Goldman Hodgkin Katz constant-field equation (also known as the GHK or Goldman equation): B Where: Na + OUT + INSIDE E = 58 [ Na ] [ Na ] log mV E = 58 460 mM 50 mM Na log mV m K + OUTNa + Cl-INSIDE K + INSIDENa Cl-OUT E = 58 P[ K ] + [ Na + ClP [ ] P [K |
|
The enduring legacy of the constant-field equation in
The Goldman current equation The starting point of the Goldman current equation is the NP equation (Eq 11) Goldman introduced the assumption of a constant electric field within the membrane (dV/dx = −ΔV/a) where ΔV is the electric potential difference across the membrane and a is the membrane thickness (Fig 2) |
Comment calculer le potentiel d'équilibre ?
Pour calculer le potentiel d'équilibre d'un ion, il y a l'équation de Nernst.
L'équation de Nernst est la suivante : Eion égale RT/zF logarithme de la concentration de l'ion à l'extérieur sur la concentration de l'ion à l'intérieur.
Eion, c'est le potentiel d'équilibre de l'ion, il s'exprime en millivolts.Comment calculer le potentiel de membrane ?
Les deux lois de Fick, du nom du physiologiste allemand Adolf Eugene Fick (1829-1901) définissent la perméabilité membranaire (P ) d'un soluté, en fonction du gradient de concentration (ΔC ) et du flux : P=J/ΔC P = J / Δ C .
Quel ion ou quelle molécule a tendance à entrer dans une cellule animale en suivant sont gradient électrochimique ?
Le gradient électrique du K+, un ion positif, le fait entrer dans la cellule, mais le gradient de concentration du K+ fait sortir le K+ de la cellule (Figure 5.16) [lien vers Biology 2e].
Nous appelons le gradient de concentration combiné et la charge électrique qui affecte un ion son gradient électrochimique.- On introduit la pointe de l'électrode dans la cellule en perforant sa membrane : la différence de potentiel est de 70mv.
Comme l'intérieur de la cellule est négatif par rapport à l'extérieur, la valeur du potentiel est de - 70 mV.
Ce potentiel dit de repos dépend du type de neurone et varie entre -40 à -90 mV.
1.1 The Resting Potential
All living cells have an electrical voltage, or potential difference, between their inside and outside. Since the cell’s membrane is what separates the inside from the outside, this potential difference is referred to as the membrane potential. In mathematical terms, the membrane potential VM is defined as VM Vin isn.ucsd.edu
Vout;
where Vin is the potential on the inside of the cell and Vout is the potential on the outside. This will change during an action potential, for example. The resting potential refers to the potential across the membrane when the cell is at rest. A typical neuron has a resting potential of about 70 mV. An inward current corresponds to a positively ch
RT ŒKC in ln
zF : ŒKC out (1.1) a Cl− Na+ Na+ Extracellular side Cytoplasmic side A− A− K+ K+ Na+ Cl− Na+ K+ A− K+ A− K+ b Cl− Na+ Na+ ++++++ - - - - - - A− A− K+ Na+ Cl− K+ Na+ ++++++ - - - - - - K+ A− K+ A− K+ Fig. 1.1 The KC flux is determined by both the KC concentration gradient and the electrical potential across the membrane. (a) For a
1.2 The Nernst Equation
Here we derive the Nernst equation and, in Sect. 1.3 we derive the GHK equation. Recall that if the membrane is permeable to only one ion, then that ion’s Nernst potential is the resting potential at which the electrical and chemical driving forces balance. The GHK equation is, in some sense, a generalization of the Nernst equa-tion in which we ass
Jdrift D
zŒC @V : @x The electric field, E @V =@x, is the gradient of the potential V (measured in volts) and thus has units of volts per centimeter. z is the valence of the ion ( ̇1; ̇2; etc.). The parameter is the mobility and has dimensions of square centimeters per volt second and ŒC is the concentration. The higher the concentration, the greater the
D D ;
q where k is Boltzmann’s constant (J/K), T is the absolute temperature, and q is the charge (measured in coulombs). Thus, we can write the total flux as Jtotal D kT @ŒC isn.ucsd.edu
@V zŒC :
q @x @x It is convenient to convert this equation, which is in terms of the number of indi-vidual molecules, into its molar equivalent, by dividing by Avogadro’s number. It is also convenient to introduce RT=F , where R is the ideal gas constant and F is Faraday’s constant, instead of kT=q. (A list of these constants is given at the end of the next
ŒC D 0: @x
@x As an exercise, it is left to the reader to prove this implies the Nernst equation: Veq Vin Vout D isn.ucsd.edu
1.3 The Goldman–Hodgkin–Katz Equation
The Nernst–Planck equation describes the movement of charged ions in aqueous media. However, the cell membrane has thickness and there may be energy barriers or blocking sites within the channel. In this case, the ions flowing through the open channel may not obey the Nernst–Planck equation and we must model the com-plex behavior within the membran
0 < x < l:
This is just a first-order linear ordinary differential equation for ŒC subject to the two boundary conditions isn.ucsd.edu
ŒC in; .l/ ŒC
ŒC out: One cannot, in general, solve a first-order equation with two boundary conditions. However, the current I is unknown, so choosing this correctly will allow us to find a solution that satisfies both boundary conditions. We leave this elementary exercise for the reader. The result is isn.ucsd.edu
RT
This expression is often written in terms of the permeability, ˇu RT P I lF that is, isn.ucsd.edu
C I.t/=A:
Note that we can rewrite this equation as dVM cM dt D isn.ucsd.edu
1 rM D gCl C gK C gNa
is the specific membrane resistance. For a passive membrane in which the conductances and currents are all constant, VM will reach a steady state: gClECl C gKEK C gNaENa C I=A isn.ucsd.edu
Vss D : gCl C gk C gNa
In the absence of the applied current, the steady-state potential is a weighted sum of the equilibrium potentials of the three currents. This is similar to the GHK equation (1.4), in which the contribution to the resting potential by each ion is weighted in proportionto the permeability of the membrane to that particular ion. Note, however, that in
1.5 The Membrane Time Constant
In this section, we consider how a passive, isopotential cell responds to an applied current. This will help explain how each component of the electrical circuit con-tributes to changes in the membrane potential. The cell is said to be passive if its electrical properties do not change during signaling. Such a cell cannot generate an action potenti
D I0 > 0;
however, this is really not necessary. Note that for an isopotential cell, the injected current distributes uniformly across the surface. It follows that for a spherical cell, the current flowing across a unit area of the membrane is I.t/ IM.t/ D D 8 isn.ucsd.edu
I0RINP;
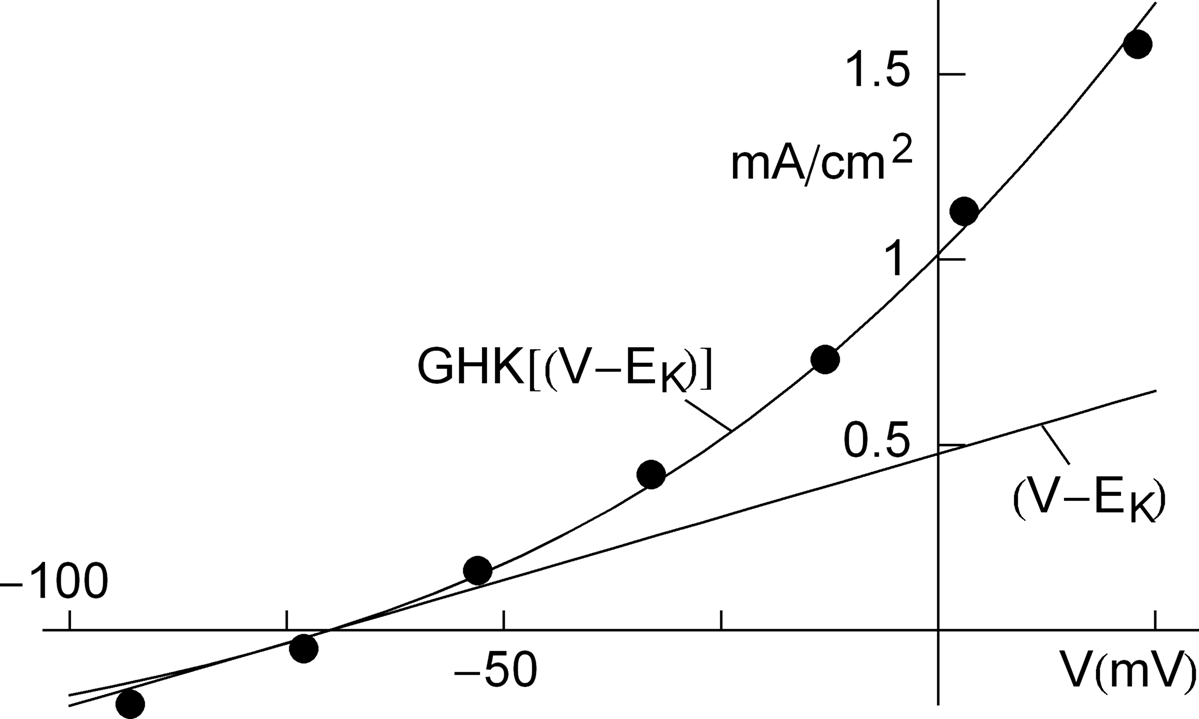
(1.18) where RINP is the input resistance of the cell. Note that if the input current changes by I , then the steady-state membrane potential changes by RINPI ; that is, the input resistance is the slope of the I–V curve obtained by plotting the steady-state voltage against the injected current. The initial rise in membrane potential is determined
1.6 The Cable Equation
We have, so far, considered the passive properties of an isopotential cell. This analysis may be used to describe signaling within the cell body, which can be ap-proximated by a sphere. However, it is clearly not appropriate for studying electrical properties of the axon or dendrites. These are better approximated by cylinders that are not isopoten
; Vh/=Vs/
where Vh and Vs are constants. We leave as an exercise the calculation of these constants in terms of the constants A and B. The time constant, .V / , will generally be a skewed bell-shaped function of V: If Bˇ , then .V / D B ̨ is a hyperbolic secant. isn.ucsd.edu
EL/:
From this equation, we can easily solve for gL. computed voltage-clamp experiment. The membrane potential is stepped from rest to 0 mV. This results in an inward current followed by an outward current. The separate KC and NaC currents are also shown IK IM INa VC = 0 mV 0 time (msec) Figure 1.8 shows the results of a (numerically computed) voltage-
1.1 The Resting Potential
All living cells have an electrical voltage, or potential difference, between their inside and outside. Since the cell’s membrane is what separates the inside from the outside, this potential difference is referred to as the membrane potential. In mathematical terms, the membrane potential VM is defined as VM Vin isn.ucsd.edu
Vout;
where Vin is the potential on the inside of the cell and Vout is the potential on the outside. This will change during an action potential, for example. The resting potential refers to the potential across the membrane when the cell is at rest. A typical neuron has a resting potential of about 70 mV. An inward current corresponds to a positively ch
RT ŒKC in ln
zF : ŒKC out (1.1) a Cl− Na+ Na+ Extracellular side Cytoplasmic side A− A− K+ K+ Na+ Cl− Na+ K+ A− K+ A− K+ b Cl− Na+ Na+ ++++++ - - - - - - A− A− K+ Na+ Cl− K+ Na+ ++++++ - - - - - - K+ A− K+ A− K+ Fig. 1.1 The KC flux is determined by both the KC concentration gradient and the electrical potential across the membrane. (a) For a
1.2 The Nernst Equation
Here we derive the Nernst equation and, in Sect. 1.3 we derive the GHK equation. Recall that if the membrane is permeable to only one ion, then that ion’s Nernst potential is the resting potential at which the electrical and chemical driving forces balance. The GHK equation is, in some sense, a generalization of the Nernst equa-tion in which we ass
Jdrift D
zŒC @V : @x The electric field, E @V =@x, is the gradient of the potential V (measured in volts) and thus has units of volts per centimeter. z is the valence of the ion ( ̇1; ̇2; etc.). The parameter is the mobility and has dimensions of square centimeters per volt second and ŒC is the concentration. The higher the concentration, the greater the
D D ;
q where k is Boltzmann’s constant (J/K), T is the absolute temperature, and q is the charge (measured in coulombs). Thus, we can write the total flux as Jtotal D kT @ŒC isn.ucsd.edu
@V zŒC :
q @x @x It is convenient to convert this equation, which is in terms of the number of indi-vidual molecules, into its molar equivalent, by dividing by Avogadro’s number. It is also convenient to introduce RT=F , where R is the ideal gas constant and F is Faraday’s constant, instead of kT=q. (A list of these constants is given at the end of the next
ŒC D 0: @x
@x As an exercise, it is left to the reader to prove this implies the Nernst equation: Veq Vin Vout D isn.ucsd.edu
1.3 The Goldman–Hodgkin–Katz Equation
The Nernst–Planck equation describes the movement of charged ions in aqueous media. However, the cell membrane has thickness and there may be energy barriers or blocking sites within the channel. In this case, the ions flowing through the open channel may not obey the Nernst–Planck equation and we must model the com-plex behavior within the membran
0 < x < l:
This is just a first-order linear ordinary differential equation for ŒC subject to the two boundary conditions isn.ucsd.edu
ŒC in; .l/ ŒC
ŒC out: One cannot, in general, solve a first-order equation with two boundary conditions. However, the current I is unknown, so choosing this correctly will allow us to find a solution that satisfies both boundary conditions. We leave this elementary exercise for the reader. The result is isn.ucsd.edu
RT
This expression is often written in terms of the permeability, ˇu RT P I lF that is, isn.ucsd.edu
C I.t/=A:
Note that we can rewrite this equation as dVM cM dt D isn.ucsd.edu
1 rM D gCl C gK C gNa
is the specific membrane resistance. For a passive membrane in which the conductances and currents are all constant, VM will reach a steady state: gClECl C gKEK C gNaENa C I=A isn.ucsd.edu
Vss D : gCl C gk C gNa
In the absence of the applied current, the steady-state potential is a weighted sum of the equilibrium potentials of the three currents. This is similar to the GHK equation (1.4), in which the contribution to the resting potential by each ion is weighted in proportionto the permeability of the membrane to that particular ion. Note, however, that in
1.5 The Membrane Time Constant
In this section, we consider how a passive, isopotential cell responds to an applied current. This will help explain how each component of the electrical circuit con-tributes to changes in the membrane potential. The cell is said to be passive if its electrical properties do not change during signaling. Such a cell cannot generate an action potenti
D I0 > 0;
however, this is really not necessary. Note that for an isopotential cell, the injected current distributes uniformly across the surface. It follows that for a spherical cell, the current flowing across a unit area of the membrane is I.t/ IM.t/ D D 8 isn.ucsd.edu
I0RINP;
(1.18) where RINP is the input resistance of the cell. Note that if the input current changes by I , then the steady-state membrane potential changes by RINPI ; that is, the input resistance is the slope of the I–V curve obtained by plotting the steady-state voltage against the injected current. The initial rise in membrane potential is determined
1.6 The Cable Equation
We have, so far, considered the passive properties of an isopotential cell. This analysis may be used to describe signaling within the cell body, which can be ap-proximated by a sphere. However, it is clearly not appropriate for studying electrical properties of the axon or dendrites. These are better approximated by cylinders that are not isopoten
; Vh/=Vs/
where Vh and Vs are constants. We leave as an exercise the calculation of these constants in terms of the constants A and B. The time constant, .V / , will generally be a skewed bell-shaped function of V: If Bˇ , then .V / D B ̨ is a hyperbolic secant. isn.ucsd.edu
EL/:
From this equation, we can easily solve for gL. computed voltage-clamp experiment. The membrane potential is stepped from rest to 0 mV. This results in an inward current followed by an outward current. The separate KC and NaC currents are also shown IK IM INa VC = 0 mV 0 time (msec) Figure 1.8 shows the results of a (numerically computed) voltage-

Nernst Potential and Goldmans Equation Nerve Physiology

Nernst Equation and Goldman Equation STEP BY STEP Nernst Potential Equilibrium Potential

Guyton chapter 5 Goldman equation summary Medical Physiology lecture 29
|
Derivation of the Goldman Equation
The Goldman equation or constant-field equation |
|
ELECTROPHYSIOLOGIE: PRINCIPES ET TECHNIQUES
Ceci aboutit à la relation courant-voltage ou équation de courant de Goldman-Hodgkin-Katz et à l'équation de potentiel pour plusieurs ions de GHK. |
|
NERNST EQUATION with GOLDMAN EXTENTION Total Force on
NERNST EQUATION with GOLDMAN EXTENTION. Total Force on an ion say K. +. = Electrical Force + Concentration (Chemical) Force. Putting in units of Voltage |
|
Lecture 6:
Lecture 1: Membrane Potential Nernst & Goldman Equations. I. Membrane Potential. A. In a typical animal cell |
|
Membrane potential profiles and the goldman equation
potential profile across a membrane in order that the Goldman equation be valid is that. This leads to the special case V(x) = V |
|
An Extension of Goldman-Hodgkin-Katz Equations by Charges from
24 août 2022 The Goldman-Hodgkin-Katz (GHK) equations have been widely applied to ion ... GHK flux equation is to treat the electric field as a known ... |
|
Application of the Goldman-Hodgkin-Katz Current Equation to
determined from the Goldman-Hodgkin-Katz voltage equation. In most cases the entire IV curve can be accounted for by using our method of analysis. |
|
Nomograms of the Goldman equation
Abstract. Solutions of the Goldman equations for sodium and potassium ions are displayed in nomograms which allow one to determine which values of the |
|
The Goldman Constant Field Assumption: Significance and
Dffusion / Membranes 1 Nernst-Planck Equations. Ionic transport phenomena in simple porous membranes can be approximately represented by the Nernst-Planck |
|
ELECTROPHYSIOLOGIE: PRINCIPES ET TECHNIQUES - ipmccnrsfr
Les équations de Goldman-Hodgkin-Katz (GHK) reposent sur plusieurs hypothèses déjà utilisées dans le modèle de l'électrodiffusion : - La membrane est homogène |
|
Rappel du dernier cours - Université de Genève
Equation de Goldman-Hodgkin-Katz (1) R Jolivet Université de Genève 19 • Sodium potassium et chlore sont les principales espèces ioniques |
|
Derivation of the Goldman Equation - Wiley Online Library
As discussed in Chapter 4 the Goldman equation describes the nonequilibrium membrane potential reached when two or more ions with unequal equilibrium |
|
Application of the Goldman-Hodgkin-Katz Current Equation to
This equation gives an explicit result for the current as a function of the concentration gradients the permeable ions present and the membrane potential as |
|
Équation de Goldman-Hodgkin-Katz en tension - Wikipédia
L'équation de Goldman-Hodgkin-Katz est une généralisation de l'équation de Nernst pour le cas d'une membrane renfermant plusieurs types de conductances |
|
3 Compartment I has a higher concentration of K - Psychology
Lecture 1: Membrane Potential Nernst Goldman Equations I Membrane Potential A In a typical animal cell potassium chloride sodium are in unequal |
|
(PDF) Thermodynamics and the Goldman-Hodgkin-Katz Equation
6 avr 2022 · Goldman-Hodgkin-Katz equation (GHK eq ) and the Nernst equation (Nernst eq ) are the typical mathematical formulas representing the membrane |
|
NERNST EQUATION with GOLDMAN EXTENTION
NERNST EQUATION with GOLDMAN EXTENTION Total Force on an ion say K + = Electrical Force + Concentration (Chemical) Force Putting in units of Voltage |
|
Box 24 The GHK equations 241 An electrical circuit approximation
Goldman (1943) and Hodgkin and Katz (1949) developed a formalism for describing the currents through and voltages across semipermeable mem- branes This |
Comment calculer le potentiel de membrane ?
Comment calculer le potentiel d'équilibre ?
. L'équation de Nernst est la suivante : Eion égale RT/zF logarithme de la concentration de l'ion à l'extérieur sur la concentration de l'ion à l'intérieur.
. Eion, c'est le potentiel d'équilibre de l'ion, il s'exprime en millivolts.
Comment calculer le potentiel de Donnan ?
. On peut calculer ainsi le potentiel d'un ion en sachant ses concentration intra et extra-cellulaire.
|
Potentiel de membrane - UNF3S
Equation fondamentale 3 3 Théorie de Attention Le signe (-) n'est plus dans l'équation, pourquoi? Equation de GOLDMANN pour les ions K+, Na+, Cl |
|
A general relation between membrane potential, ion activities, and
The Goldman equation as originally derived [ 1,2] depended on the assumptions of a constant field across the membrane, zero net current flow across the |
|
Application of the Goldman-Hodgkin-Katz Current Equation to
calculated permeability coefficients are in agreement with those ratios determined from the Goldman-Hodgkin-Katz voltage equation In most cases, the entire IV |
|
Goldman-Hodgkin-Katz Equations
The GHK current equation gives the transmembrane current of an ion species expected at a given membrane potential for a given concentration of the ion on |
|
Lecture 6:
Lecture 1: Membrane Potential, Nernst Goldman Equations I Membrane Potential 1 If you substitute these equations in the simple equation at equilibrium: |
|
Introduction to Computational Neuroscience Biol 698 Math - NJIT
The passive membrane equation (review) • Goldman-Hodgkin-Katz equation • Mechanism of action potential generation • Hodgkin-Huxley equation |
|
Model neurons - TU Chemnitz
Nenst equation • Goldman-Hodgkin-Katz equation • Ion concentrations • Membrane capacity • RC-circuit Model neurons: The membrane equation |





![David E Goldman ○ 1910–1998 - [PDF Document] David E Goldman ○ 1910–1998 - [PDF Document]](https://0.academia-photos.com/attachment_thumbnails/38653867/mini_magick20190224-27387-uqs8fq.png?1551035626)