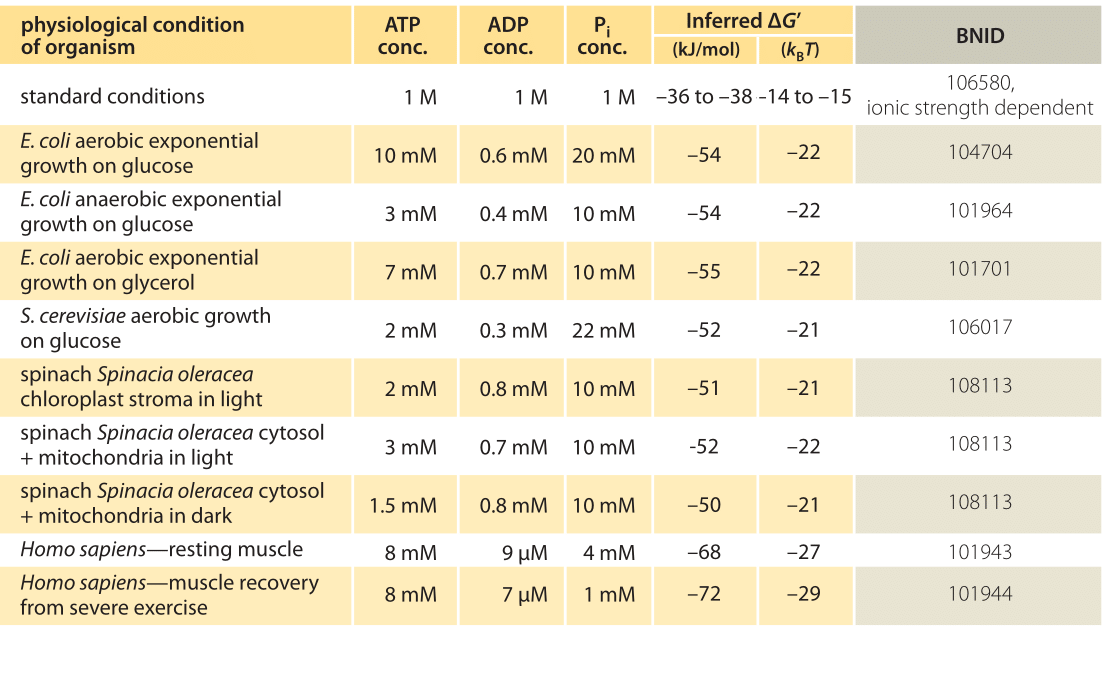
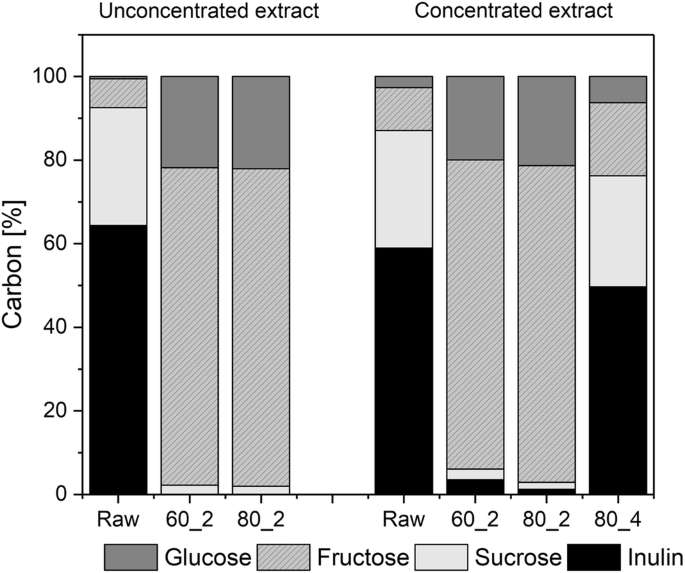
equilibrium constant for hydrolysis of sucrose
Why do we need equilibrium constants for a hydrolysis reaction?
Consequently, it is necessary to determine equilib- rium constants (i.e. Gibbs energy changes) for a series of reactions which can be algebraically combined to yield a value of the equilibrium constant for the hydrolysis reaction.
What is the equilibrium constant for aqueous sucrose?
The equilibrium constants determined at 298.15 K for processes B and C are 17.1 ± 1.0 and 32.4 ± 3.0, respectively. Equilibrium data for process D was obtained from the literature, and in conjunction with the data for processes B and C, used to calculate a value of the equilibrium constant for the hydrolysis of aqueous sucrose.
How enthalpy change the hydrolysis of sucrose?
The enthalpy change the hydrolysis of sucrose (process for A) can also be compared with the value calculated using the enthalpy of combustion (19) and enthalpy of solution (20) of sucrose (cr) and the enthalpiesformation of aqueous glucose of and fructose (12). The resulting value is - (16.04 f 1.5) kJ mol".
How is the hydrolysis of sucrose to fructose and glucose performed?
A thermodynamic investigation of the hydrolysis of sucrose to fructose and glucose has been performed using microcalorimetry and high-pressure liquid chromatography. The calorimetric measurements were carried out over the temperature range 298–316 K and in sodium acetate buffer (0.1 M, pH 5.65).

s=

Hydrolysis of sucrose gives `Sucrose +H_(2)OhArrGlucose + Fructose` Equilibrium constant `K_(c)`

Hydrolysis of sucrose gives Sucrose + H2O ⇌ Glucose + Fructose
|
Thermodynamics of the hydrolysis of sucrose
15 juin 2006 a value of the equilibrium constant for the hydrolysis of aqueous sucrose. ... The hydrolysis of sucrose to glucose and fructose is cata-. |
|
A calorimetric and equilibrium investigation of the hydrolysis of lactose
8 févr. 1989 has less sweetening capacity than sucrose and is also less soluble in water. ... In principal values of the equilibrium constant and. |
|
M.SC PHYSICAL CHEMISTRY PRACTICAL MANUAL
Acid-catalyzed hydrolysis of sucrose (inversion of sucrose). Adsorption and others: Determination of the hydrolysis constant of aniline hydrochloride. |
|
Reaction Kinetics and Modeling of the Enzyme-catalyzed Production
be expressed by an equilibrium constant (the rapid equilibrium assumption). and k is the sucrose hydrolysis reaction rate constant defined as k4/K§. |
|
Inversion of Sucrose1 Purpose: The rate of reaction between
hydrolysis of sucrose into single sugars is necessary before the sugars can be also use a large excess of acid to maintain a constant hydrogen ion ... |
|
THE INFLUENCE of pH ON the KINETICS of ACID HYDROLYSIS of
Sucrose acid hydrolysis was studied as a potential chemical time- slowest thus the rate-determining step |
|
Real-time benchtop NMR spectroscopy for the online monitoring of
20 nov. 2020 this study sucrose hydrolysis by invertase was chosen as a model ... kinetic constants determined by the fractional conversion model were ... |
|
The oxygen-18 isotope shift in carbon-13 nuclear magnetic
While the equilibrium ratio of 180 was detected The acid-catalyzed hydrolysis of sucrose has a rich history. It ... The rate constant of the. |
|
Kinetic solvent effects on acid-catalyzed hydrolysis of sucrose in
Rate constants of acid-catalyzed hydrolysis of sucrose (S) to D-glucose and L-fructose have been determined solvent mixtures postulate the equilibrium. |
|
Kinetic Studies of Levansucrase of Bacillus subtilis
The initial velocity of levan hydrolysis was tion to sucrose concentration was kept constant and equal to the equilibrium constant. After 10 min. |
|
Thermodynamics of the Hydrolysis of Sucrose* - ResearchGate
15 jui 2006 · II Formerly National Bureau of Standards hydrolysis of disaccharides which had indicated that the en- tropy changes for the hydrolysis of disaccharides were reason- ably constant and ranged from 30 to 43 J mol” K-' |
|
Inversion of Sugar : The hydrolysis of sucrose by boiling with a
While carbohydrates exist almost entirely as cyclic hemiacetals in aqueous solutions, they are in rapid equilibrium Sucrose solution is dextro rotatory but during hydrolysis it becomes The constant value of k shows that the reaction is of first |
|
STUDIES ON THE PHOSPHOROLYSIS OF SUCROSE - ScienceDirect
phorolysis and hydrolysis of sucrose could readily be extracted from the dry cells with an apparent equilibrium constant may be derived for the phosphorolytic |
|
The Equilibrium ofthe Reaction Catalyzed by Sucrose - CORE
The equilibrium constant for the reaction catalyzed by sucrose phos- phate synthase was reported 25 years ago to some hydrolysis ofUDP to UMP was noted |
|
Reaction Kinetics and Modeling of the Enzyme-catalyzed Production
The reaction rate constants, equilibrium constant, and dissociation and Michaelis constants were and k) is the sucrose hydrolysis reaction rate constant |