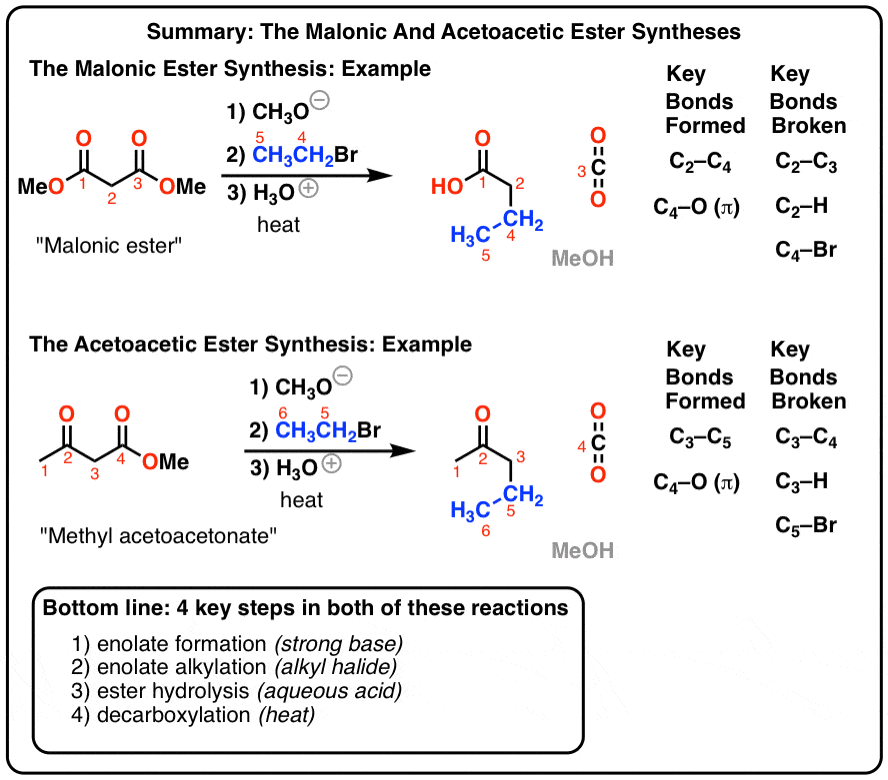
types of hydrolysis
|
Techno-economic and life cycle analysis of different types of
TYPES OF HYDROLYSIS PROCESS FOR THE PRODUCTION OF P-. XYLENE. Abhay Athaley. 1. Praneeth Annam |
|
Continuous hydrolysis of milk proteins in membrane reactors of
31 авг. 2021 г. Continuous MBRs are the most economically profitable type as they are based on the difference in molecular weight between the enzyme and the ... |
|
A DESCRIPTION OF HYDROLYSIS KINETICS IN ANAEROBIC
Four types of hydrolysis kinetics were tested for anaero- t bic degradation of complex organic matter using the. X generalized simulation model described |
|
Hydrolysis of suxamethonium by different types of plasma
Rates of hydrolysis of butyrylcholine 10 mm |
|
In vitro antioxidant and wound-healing activities of hydrolyzed
19 мая 2021 г. 4 Apart from the type of enzyme used the hydrolysis sequence also affected the size as well as bioactivities of peptides obtained. For example |
|
Comparison of the Hydrolysis of the Three Types of Natriuretic
Comparison of the Hydrolysis of the Three Types of Natriuretic. Peptides by Human Kidney Neutral Endopeptidase 24.11. Yasuhiro Watanabe Kenjiro Nakajima |
|
Effect of Acid And Hydrolysis Duration on The Characteristics of
There were group of treatments including source of starch: arrowroot and taro beneng starch and the acid types was HCland H2SO4 |
|
Hydrolysis of microcrystalline cellulose isolated from waste seeds of
14 апр. 2019 г. The aim of this work was to produce glucose from the microcrystalline cellulose (MCC) obtained from two types of waste. Leucaena leucocephala ... |
|
HYDROLYSIS 2016.pdf
Primary carbamates (R1 or R2 = H) undergo hydrolysis via an E1cB type mechanism much faster than secondary carbamates which hydrolyze via a. BAC2 mechanism. N. |
|
ENZYMATIC HYDROLYSIS OF SOY PROTEIN FOR NUTRITIONAL
Different types of centrifuges were tried for the sepa- rations; generally of hydrolysis curves for a number of proteins hydrolyzed with Alcalase shown ... |
|
HYDROLYSIS 2016.pdf
Primary carbamates (R1 or R2 = H) undergo hydrolysis via an E1cB type mechanism much faster than secondary carbamates which hydrolyze via a. BAC2 mechanism. N. |
|
Effect of Acid And Hydrolysis Duration on The Characteristics of
There were group of treatments including source of starch: arrowroot and taro beneng starch and the acid types was HCland H2SO4 |
|
Techno-economic and life cycle analysis of different types of
Lignocellulosic biomass is used as the starting material for all the three hydrolysis process with different initial loading. Sensitivity analysis is carried |
|
Two Types of Lipases in Hydrolysis of Polyester
R. delemar lipase could hydrolyze the polyester moiety of polyurethane which hog pancreas there are two types of lipases in the hydrolysis. |
|
Polymer link breakage of polyimide-film-surface using hydrolysis
This chemical decomposition was performed through two types of hydrolysis reactions that is |
|
The effect of the type of hydrolysis of aluminum coagulants on the
Among the tested coagulants the pre-hydrolyzed. PAXXL10 coagulant provided the greatest efficiency of total organic carbon removal from water. Aluminum sulfate |
|
Production of Low Molecular Weight Chitosan and
27 juil. 2021 Enzymatic hydrolysis is the only method that has association restrictions with other types of hydrolysis due to the sensitivity of the enzymes ... |
|
Application of hydrolysis probe analysis to identify clade types of the
18 sept. 2017 A hydrolysis probe analysis (TaqMan assay) was used to study clade types in Anopheles funestus sensu stricto Giles a major malaria vector ... |
|
A theoretical comparison of the reactors for the enzymatic hydrolysis
Accepted for publication September 2 1986. A reactor's design is of great importance in the en- zymatic hydrolysis of cellulose. Three types of reac-. |
|
Bioethanol Production by Enzymatic Hydrolysis from Different
1 févr. 2021 Lignocellulosic biomass includes all kinds of agricultural wastes forestry residues |
|
Hydrolysis: Definition examples Types- salt ATP Applications
Hydrolysis are generally enhanced by both acids and bases and three independent reaction mechanisms account for neutral acid and base hydrolysis The overall hydrolysis kinetics has three contributing components Rate of hydrolysis = kh [RX] where kh = kA[H +] + k N + kB[OH-] |
What Is hydrolysis?
Hydrolysis is derived from a Greek word hydro meaning water and lysis which translates to the word break or to unbind. Usually in hydrolysis the water molecules get attached to two parts of a molecule. One molecule of a substance will get H+ ion and the other molecule receives the OH- group. Hydrolysis reaction is mainly used to break down polymers...
Types of Hydrolysis
There are several types of hydrolysis, and we will look at them in brief below. 1. 1.1. 1.1.1. 1.1.1.1. Salts:This is the most common type of hydrolysis. Hydrolysis of salts generally refers to the reaction of salt with water where it involves the interaction between cations or anions of salts and water. During hydrolysis, a salt breaks down to for...
What are the three types of hydrolysis?
Hydrolysis is of three types- salts hydrolysis, acid and base hydrolysis, ATP hydrolysis. Let us discuss about them in detail- Salt hydrolysis is one of the most common hydrolysis. When salt reacts with water, there is cation anion interaction between salt and water.
What are the three types of hydrolysis of ammonia?
In the hydrolysis of ammonia, ammonia reacts with water to form ammonium and hydroxide ion. The chemical reaction for the same is as follows- Hydrolysis is of three types- salts hydrolysis, acid and base hydrolysis, ATP hydrolysis. Let us discuss about them in detail- Salt hydrolysis is one of the most common hydrolysis.
What is the rate of hydrolysis?
=t Hydrolysis are generally enhanced by both acids and bases and three independent reaction mechanisms account for neutral, acid and base hydrolysis. The overall hydrolysis kinetics has three contributing components. Rate of hydrolysis = kh[RX] where, kh= kA[H +] + k N+ kB[OH NUCLEOPHILIC SUBSTITUTION/ELIMINATION MECHANISM
What does hydrolysis mean in chemistry?
Literally, hydrolysis implies water reaction. It is a chemical mechanism in which, by adding a molecule of water, a molecule is broken into two pieces. As a salt with a weak acid or weak base (or both) is dissolved in water, the most common hydrolysis happens. What is hydrolysis used for?
|
HYDROLYSIS
In general, hydrolysis occurs via one of two classes of mechanisms; i) Nucleophilic Substitution (SN1 and SN2), generally occurs when the leaving group is attached to sp3 hybridized carbon centre, such as alkyl halides, epoxides and phosphate esters |
|
Lecture 6: Hydrolysis Reactions of Esters and Amides
draw the mechanism of ester hydrolysis under acidic and basic reaction conditions; Other types of alkyl esters undergo hydrolysis via different reaction |
|
5 Design and Economic Evaluation of Cellulose Hydrolysis Processes
Two types of reactors, a batch reactor and a continuously stirred tank reactor ( CSTR), are considered The economic optimization has entailed the determination of |
|
Pretreatment and Process Hydrolysis
Type Title/Performance Measure Due Date Regular Title: Determine Enzymatic Hydrolysis Glucose and Xylose Yields of DDR solids at High Solids Loadings |
|
Complete chemical hydrolysis of cellulose into fermentable sugars
that affect the hydrolysis reaction include the type of cellulose substrate, the type of IL hemicellulose and lignin, is first broken down and hydrolyzed into simple |
:max_bytes(150000):strip_icc()/what-is-hydrolysis-375589-v2-5bdb527846e0fb002d706279.png)