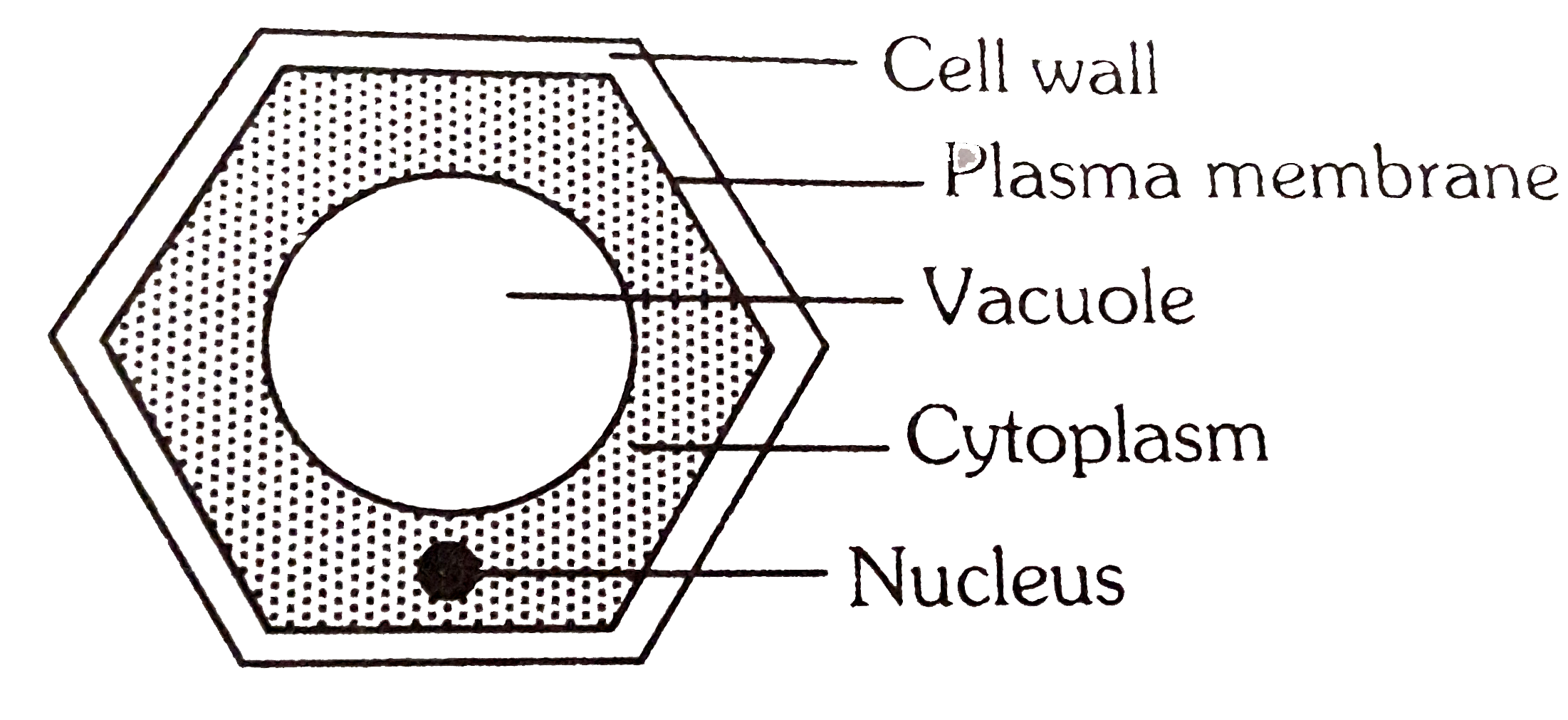animal cell immersed in hypotonic solution
Why do plants have a defense against a hypotonic solution?
In this case, that is the inside of the cell. A cell in a hypotonic solution may gain enough water to lyse, or rupture, the cell membrane, which destroys the cell. Plant cells have some defense against this phenomenon because their cell walls prevent the cell from rupturing.
Overview
Osmosis and tonicity. Hypertonic, isotonic, and hypotonic solutions and their effect on cells. khanacademy.org
Introduction
Have you ever forgotten to water a plant for a few days, then come back to find your once-perky arugula a wilted mess? If so, you already know that water balance is very important for plants. When a plant wilts, it does so because water moves out of its cells, causing them to lose the internal pressure—called turgor pressure—that normally supports the plant. Why does water leave the cells? The amount of water outside the cells drops as the plant loses water, but the same quantity of ions and other particles remains in the space outside the cells. This increase in solute, or dissolved particle, concentration pulls the water out of the cells and into the extracellular spaces in a process known as osmosis. khanacademy.org
How it works
Why does water move from areas where solutes are less concentrated to areas where they are more concentrated? This is actually a complicated question. To answer it, let’s take a step back and refresh our memory on why diffusion happens. In diffusion, molecules move from a region of higher concentration to one of lower concentration—not because they’re aware of their surroundings, but simply as a result of probabilities. When a substance is in gas or liquid form, its molecules will be in constant, random motion, bouncing or sliding around one another. If there are lots of molecules of a substance in compartment A and no molecules of that substance in compartment B, it’s very unlikely—impossible, actually—that a molecule will randomly move from B to A. On the other hand, it’s extremely likely that a molecule will move from A to B. You can picture all of those molecules bouncing around in compartment A and some of them making the leap over to compartment B. So, the net movement of molecules will be from A to B, and this will be the case until the concentrations become equal. In the case of osmosis, you can once again think of molecules—this time, water molecules—in two compartments separated by a membrane. If neither compartment contains any solute, the water molecules will be equally likely to move in either direction between the compartments. But if we add solute to one compartment, it will affect the likelihood of water molecules moving out of that compartment and into the other—specifically, it will reduce this likelihood. Why should that be? There are some different explanations out there. The one that seems to have the best scientific support involves the solute molecules actually bouncing off the membrane and physically knocking the water molecules backwards and away from it, making them less likely to cross1,2 . Regardless of the exact mechanisms involved, the key point is that the more solute water contains, the less apt it will be to move across a membrane into an adjacent compartment. This results in the net flow of water from regions of lower solute concentration to regions of higher solute concentration. This process is illustrated in the beaker example above, where there will be a net flow of water from the compartment on the left to the compartment on the right until the solute concentrations are nearly balanced. Note that they will not become perfectly equal in this case because the hydrostatic pressure exerted by the rising water column on the right will oppose the osmotic driving force, creating an equilibrium that stops short of equal concentrations. khanacademy.org
Osmolarity
Osmolarity describes the total concentration of solutes in a solution. A solution with a low osmolarity has fewer solute particles per liter of solution, while a solution with a high osmolarity has more solute particles per liter of solution. When solutions of different osmolarities are separated by a membrane permeable to water, but not to solute, water will move from the side with lower osmolarity to the side with higher osmolarity. Three terms—hyperosmotic, hypoosmotic, and isoosmotic—are used to describe relative osmolarities between solutions. For example, when comparing two solution that have different osmolarities, the solution with the higher osmolarity is said to be hyperosmotic to the other, and the solution with lower osmolarity is said to be hypoosmotic. If two solutions have the same osmolarity, they are said to be isoosmotic. khanacademy.org
Tonicity
In healthcare settings and biology labs, it’s often helpful to think about how solutions will affect water movement into and out of cells. The ability of an extracellular solution to make water move into or out of a cell by osmosis is known as its tonicity. Tonicity is a bit different from osmolarity because it takes into account both relative solute concentrations and the cell membrane’s permeability to those solutes. Three terms—hypertonic, hypotonic, and isotonic—are used to describe whether a solution will cause water to move into or out of a cell: If a cell is placed in a hypertonic solution, there will be a net flow of water out of the cell, and the cell will lose volume. A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. If a cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume. If the solute concentration outside the cell is lower than inside the cell, and the solutes cannot cross the membrane, then that solution is hypotonic to the cell. khanacademy.org
Tonicity in living systems
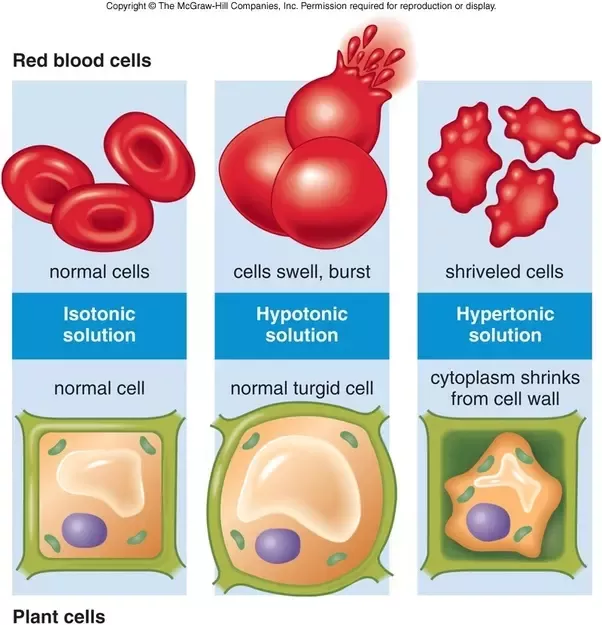
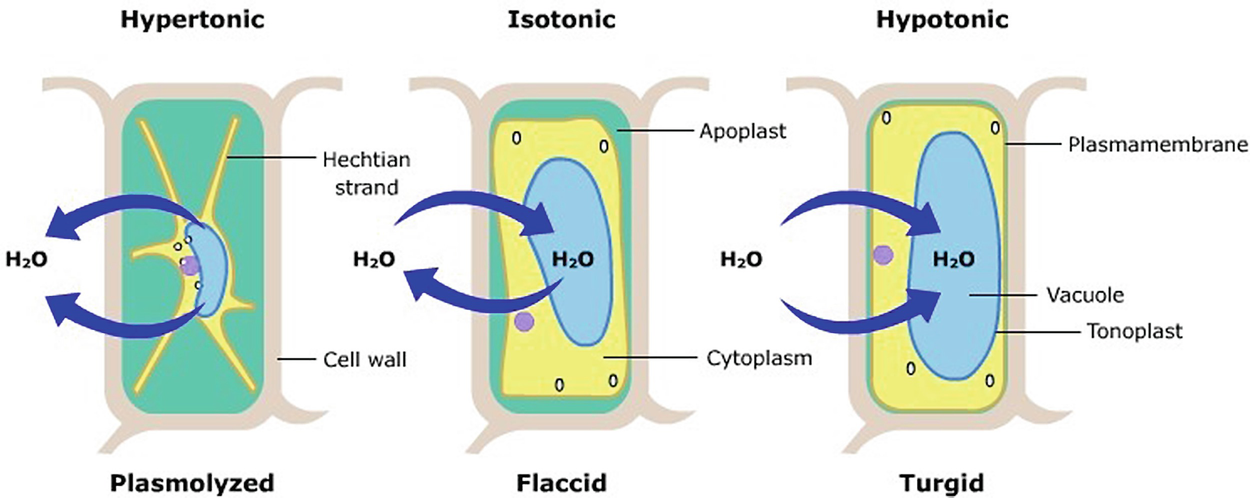
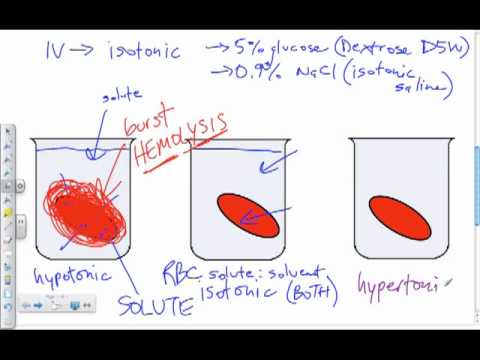
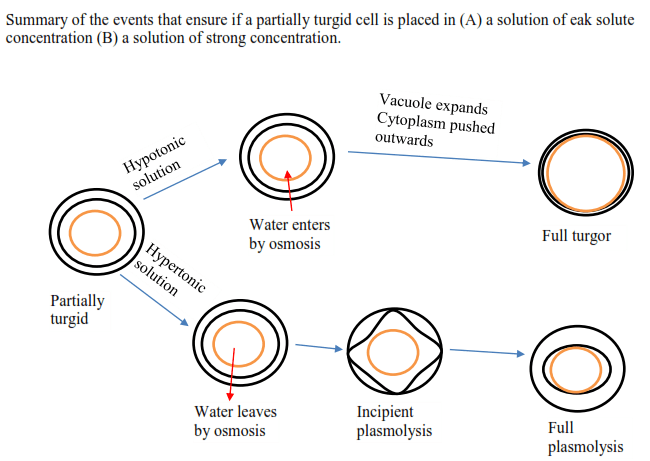
If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. In an isotonic environment, there is no net water movement, so there is no change in the size of the cell. When a cell is placed in a hypotonic environment, water will enter the cell, and the cell will swell. In the case of a red blood cell, isotonic conditions are ideal, and your body has homeostatic (stability-maintaining) systems to ensure these conditions stay constant. If placed in a hypotonic solution, a red blood cell will bloat up and may explode, while in a hypertonic solution, it will shrivel—making the cytoplasm dense and its contents concentrated—and may die. In the case of a plant cell, however, a hypotonic extracellular solution is actually ideal. The plasma membrane can only expand to the limit of the rigid cell wall, so the cell won't burst, or lyse. In fact, the cytoplasm in plants is generally a bit hypertonic to the cellular environment, and water will enter a cell until its internal pressure—turgor pressure—prevents further influx. Maintaining this balance of water and solutes is very important to the health of the plant. If a plant is not watered, the extracellular fluid will become isotonic or hypertonic, causing water to leave the plant's cells. This results in a loss of turgor pressure, which you have likely seen as wilting. Under hypertonic conditions, the cell membrane may actually detach from the wall and constrict the cytoplasm, a state called plasmolysis (left panel below). Tonicity is a concern for all living things, particularly those that lack rigid cell walls and live in hyper- or hypotonic environments. For example, paramecia—pictured below—and amoebas, which are protists that lack cell walls, may have specialized structures called contractile vacuoles. A contractile vacuole collects excess water from the cell and pumps it out, keeping the cell from lysing as it takes on water from its hypotonic environment. [Attribution and references] khanacademy.org
Want to join the conversation?
Log in khanacademy.org
|
A Cell In A Hypertonic Solution Will - web.mei.edu
An animal cell which is placed in a hypertonic solution loses water by immersed in distilled water (hypotonic solution) and salty water (hypertonic ... |
|
(From The Biological Laboratory Cold Spring Harbor
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1394301/pdf/jphysiol01600-0055.pdf |
|
Relation between Water Permeability and Integrity of Sulfhydryl
A number of malignant human and animal cell (Merthiolate) gave rise to scallop blebs. FIGURE 3. Symmetrical blebs. Sarcoma 37 ascites cells after immersion in ... |
|
Introduction Fixation is an attempt at stabilizing biological systems
Most animal cells cell if the cell neither swells nor shrinks when immersed in it. Hypertonic solutions cause shrinkage while hypotonic solutions induce ... |
|
IB Questionbank
Deduce with a reason |
|
TRANSPORT IN CELLS
What is different about a plant cell placed in a hypotonic solution and an animal cell? that it is constantly immersed in a hypotonic solution. Paramecium ... |
|
Untitled
An artificial cell consisting of an aqueous solution enclosed in a selectively permeable membrane has just been immersed in a beaker containing a different |
|
An Experimental Study on the Structure of Living Nuclei in the
By the immersion in a more highly hypertonic solution however |
|
Exercise 15
When cells are immersed in hypertonic solution shrinkage of protoplasm takes place with visible separation of plasma membrane from the cell walls. This is |
|
454 + 19 ml./kg and 2-36 + 0-05 x 10-6 cm sec at 370 C. cell and the
also found that taeniae coli immersed in hypertonic DMSO solution (1.4 M) at hypotonic solutions can diffusion in the cell interior affect the measure-. |
|
TRANSPORT IN CELLS
Transport in Cells B1YvM2. 4. MODEL 3: Osmosis in Plant and Animal Cells. External solution: Hypertonic. Isotonic. Hypotonic. Animal. Cell. |
|
Untitled
In a hypotonic solution animal cells experience osmosis and a pressure builds up in the cell. What causes the pressure? (Be specific). Hypotonic solution =. |
|
BIOL 347L Laboratory Three
Tonicity is commonly used when describing the response of cells immersed in an A Hypotonic solution has less solute (so MORE water) than the cell. |
|
BIOL 305L Laboratory Two
Tonicity is commonly used when describing the response of cells immersed in an external solution. Like osmotic pressure tonicity is influenced only by |
|
Introduction Fixation is an attempt at stabilizing biological systems
Tonicity. A solution is said to be isotonic with a cell if the cell neither swells nor shrinks when immersed in it. Hypertonic solutions cause shrinkage |
|
Osmosis-in-onion-cells.pdf
In the distilled water solution what environment was this cell in: hypertonic |
|
Exercise 15
When cells are immersed in hypertonic solution shrinkage of protoplasm takes place with visible Different stages of plasmolysis in a plant cell. |
|
Water Potential (?)
When the pressure exerted outward on the water surrounding the plant cell is equal to the osmotic potential of the solution in the cell the water potential |
|
Cellular Transport Review-kEY.pdf
When water leaves a plant cell the osmotic pressure will . increase decrease. The shrinking of ANIMAL cells that are placed in a HYPERTONIC solution is called |
|
LAB TWO
A cell placed in this solution will give up water (osmosis) and shrink A Hypotonic solution has less solute (so MORE water) than the cell A cell placed in this solution will take up water (osmosis) and expand A cell placed in this solution will stay the same |
|
TRANSPORT IN CELLS
Schematic Diagram of Transport of Water in a Sugar Solution: For each question use What is different about a plant cell placed in a hypotonic solution and an animal cell? 21 Considering the constantly immersed in a hypotonic solution |
|
Part 1 Osmosis Practice Key - Mayfield City Schools
Label the type of solution hypertonic, hypotonic, or isotonic on the line below the picture 3 Explain what happens to animal cells if they are put in fresh water |
|
Unit_5_answer_key_hbiopdf
Animal Cell (blood cell) Environment Before After Before After Isotonic solution: same Same drawing shriveleti drawing Hypertonic Solution Shrivera |
|
Unit 3 ws keypdf - Humble ISD
An artificial cell, containing a solution enclosed in a selectively permeable membrane, has just been immersed in a beaker containing a different solution Happens to an animal cell in a hypertonic solution _23 Requires ATP to move |
|
PLANT CELLS & OSMOSIS - Esalq
Thus, a hypotonic solution has more water than the cell and water has a tendency to move (diffuse) into the cell Placing Elodea cells into 100 water, which is |
|
Answers here - JBS Science Department - John Burroughs School
O- Animal coll 1 - Plant_all cell B m hypotonic 20 solute 20 solute of an aqueous solution enclosed in a selectively permeable membrane is immersed |
|
Diffusion Practice 2 answers Bpdf - Madeira City Schools
from hypotonic to hypertonic solution Cell is immersed in solution of sucrose and glucose whose individual A plant cell is placed in a beaker of pure water |
|
Osmosis under the microscope - GTAC
When cells are bathed in a solution where the solute concentration is higher than Figure 1 Effects of hypertonic, isotonic and hypotonic solutions on plant cells |