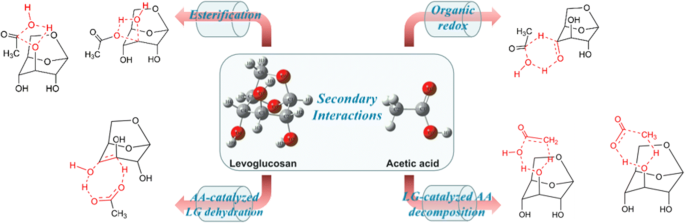
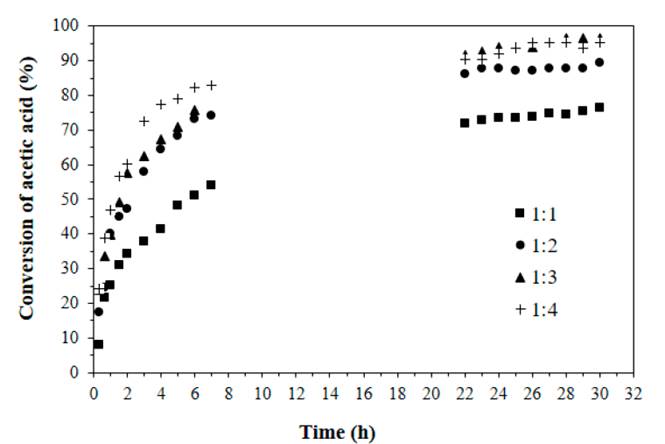
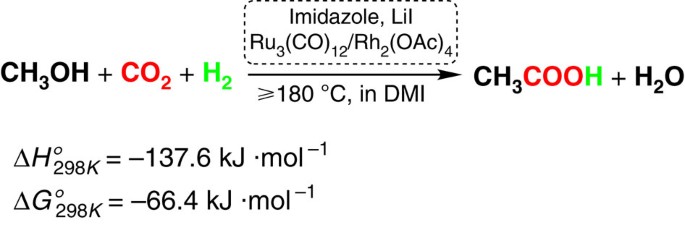
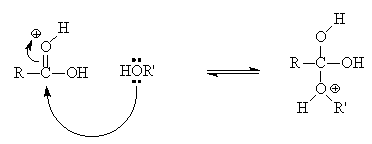
ethanoic acid esterification mechanism
What is reversible esterification?
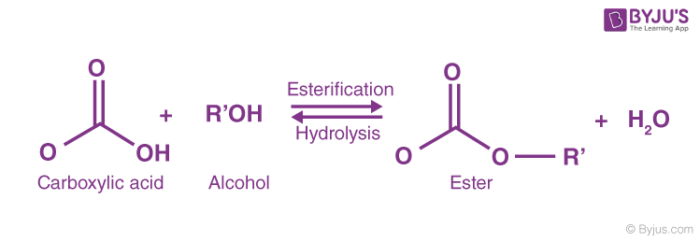
To identify and describe the substances from which most esters are prepared. Some esters can be prepared by esterification, a reaction in which a carboxylic acid and an alcohol, heated in the presence of a mineral acid catalyst, form an ester and water: The reaction is reversible.
What is the reaction between alcohols and carboxylic acids to make esters?
This page looks at esterification - mainly the reaction between alcohols and carboxylic acids to make esters. It also looks briefly at making esters from the reactions between acyl chlorides (acid chlorides) and alcohols, and between acid anhydrides and alcohols. Esters are derived from carboxylic acids.
What Is esterification?
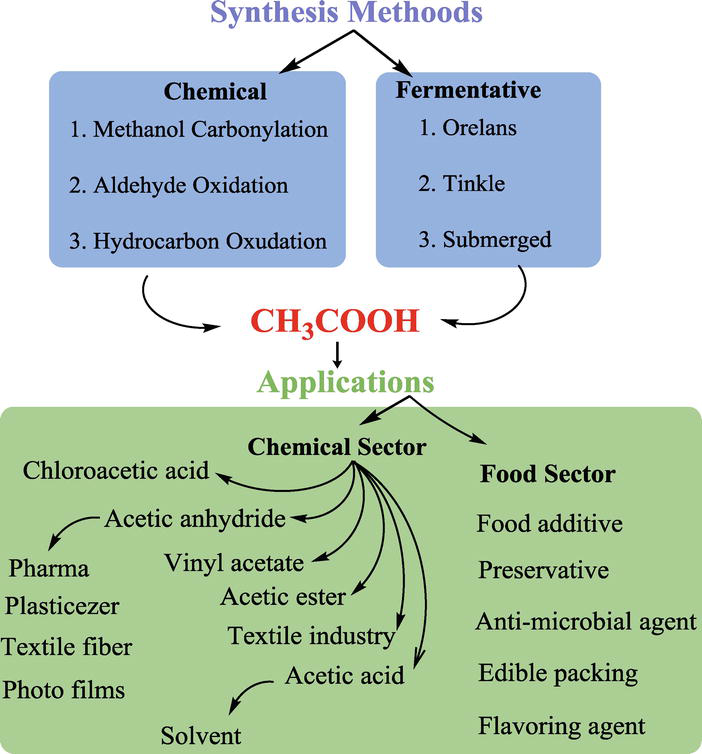
Esterification is the process of combining an organic acid (RCOOH) with an alcohol (ROH) to form an ester (RCOOR)and water; or a chemical reaction resulting in the formation of at least one ester product. Ester is obtained by an esterification reaction of an alcohol and a carboxylic acid. byjus.com
Table of Contents
What is Esterification Reaction?Esterification MechanismEsterification MethodsProperties of Esters byjus.com
What Is Esterification reaction?
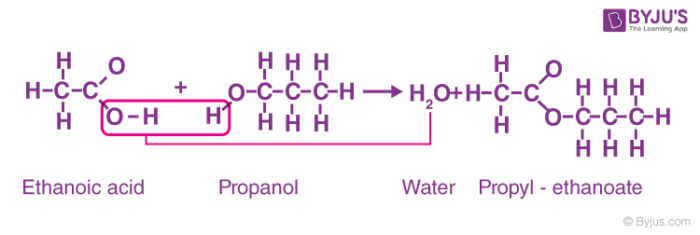
When primary alcohol is treated with a carboxylic acid in the presence of sulphuric acid a compound is formed. This compound has a sweet smell. The compound obtained is called ester. The chemical reactionoccurring in the formation of the ester is known as an esterification reaction. CH3COOH + CH3CH2COOH → CH3COOCH2CH3 byjus.com
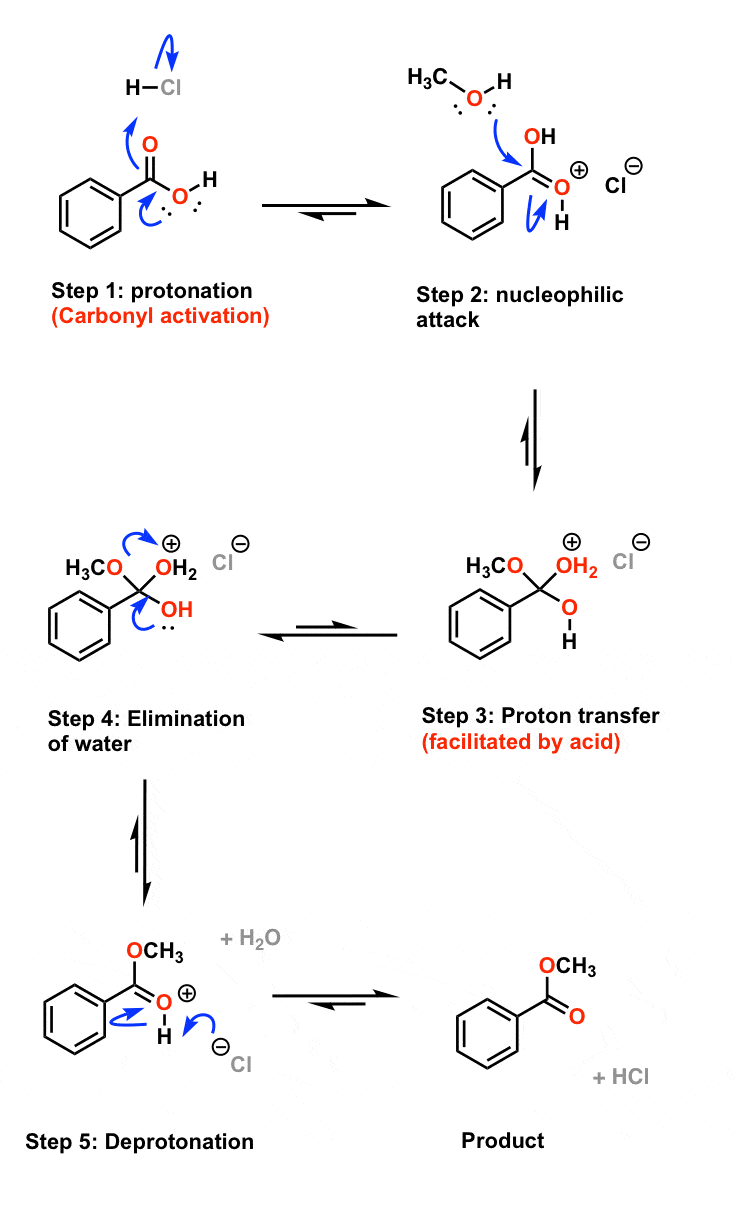
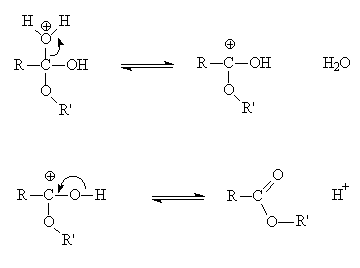
Esterification Mechanism
This process involves five steps. We have discussed the steps below: Step 1:Cation formation Step 2:Delocalized carbocation Carboxyl oxygen gets protonated to give delocalized carbocation making the carbocation a better electrophile. Step 3:Transfer of proton A proton is transferred to one of the hydroxyl groups to form a good leaving group. Step 4
Esterification Methods
Esterification can happen in three ways. They are discussed below: 1. From acid anhydride and alcohol 2. From acid chloride and alcohol 3. From carboxylic acid and alcohol byjus.com
Properties of Esters
The name of an ester is derived from its carboxylic acid that takes part in the esterification reaction.They have a pleasant smell.They have a wide application in the perfume and food industries.Esters are organic compounds found in oils and fats. byjus.com
Uses of Esters
Some common uses of esters are mentioned below. 1. Esters that have fragrance are used in perfumes, food flavourings, and cosmetics. 2. Used as an organic solvent. 3. Nitroglycerin is known and famous for its explosive properties. 4. In the manufacturing of surfactants like detergents and soaps. 5. Natural occurring esters are present in pheromones

Properties of Ethanoic Acid: Esterification reaction

Fischer Esterification Reaction Mechanism

Ethanoic Acid + Ethanol = ?? (Ester Reaction)
|
Chapter 5 Carboxylic Acids and Esters
Learn the major chemical reaction of carboxylic acids and esters and learn how to Ethanoic acid/acetic acid is the main ingredient in vinegar. |
|
PicoSpin™ 45: The Fisher Esterification Reaction Synthesis of
The purpose of this experiment is to synthesize isopentyl acetate (3-methylbutyl acetate) via an esterification reaction between acetic acid and isopentyl |
|
10. Fisher Esterification: Preparation of Banana Oil
The overall mechanism for a general acid and alcohol is depicted in Figure 2. isopentyl alcohol (3-methyl-1-butanol) and acetic acid (ethanoic acid ... |
|
ESTERIFICATION OF ACETIC ACID AND BENZYL ALCOHOL
The acetic acid esterification follows the Eley-Rideal mechanism with the conversion improved by prolonging the reaction time and increasing the amount of |
|
Research paper determination of adsorption and kinetic parameters
reaction was found to be 45.4kJ mol? 1. KEYWORDS: butyl acetate acetic acid |
|
Insights from OH radical oxidation of acetic acid
2011. jún. 28. Please refer to the corresponding final paper in ACP if available. Mechanisms leading to oligomers and. SOA through aqueous photooxidation:. |
|
8-Synthesis-of-Aspirin.pdf
The phenol group on the salicylic acid forms an ester with the carboxyl group on the acetic acid. However this reaction is slow and has a relatively low yield. |
|
Kinetics of esterification of acetic acid and methanol using Amberlyst
CH3COOH+CH3OH?CH3COOCH3+H2O. (1). Catalytic esterification of acetic acid with methanol. Progress in Reaction Kinetics and Mechanism 2015 |
|
Reaction Kinetics and Mechanism for the Gas- and Liquid-Phase
A comprehensive kinetic investigation of the esterification of acetic acid with methanol in both the liquid phase (21-60 °C) and the gas phase (100-140 °C) |
|
Research paper kinetic study of the esterification of acetic acid with 1
The uncatalysed. Page 2. reaction was shown to proceed by a second-order mechanism. In the presence of the catalyst the reaction was found to occur between an |
|
Kinetics and Mechanism of Ethyl Acetate Production Using Eco
11 jui 2016 · mechanism Introduction Esterification reaction generally refers to the formation of esters by the interaction of alcohols and carboxylic acids |
|
Worksheet 1
Esters are produced by the reaction between alcohols and carboxylic acids For example, reacting ethanol with acetic acid to give ethyl acetate is shown below → |
|
The Mechanism of the Gas-Phase Esterification of Acetic Acid and
Esterification reactions are conventionally carried out using ho- mogeneous acids, such as sulphuric acid Especially in view of catalyst separation, the use of |
|
Kinetic Study of Esterification Reaction - CORE
The Esterification kinetics of acetic acid with ethanol in the presence of sulfuric acid as a homogenous catalyst was studied with isothermal batch experiments at |
|
Esterification of Benzyl Alcohol with Acetic Acid over - CORE
Received: 21st November 2016; Revised: 1st February 2017; Accepted: 18th February 2017 Page 2 Bulletin of Chemical Reaction Engineering Catalysis, |
|
Esterification of acetic acid and benzyl alcohol over Zeolite - ukmmy
In this study, the performance of zeolite HX synthesized from kaolin was investigated as acid catalyst in esterification reaction The activity was also compared to |
|
By- step reaction pathway for formation of an ester from ac
4 8 Reaction Pathway for Esterification A somewhat simplified step- ester from a carboxylic acid and an alcohol is shown below Kirt Michael, Organic Chem |














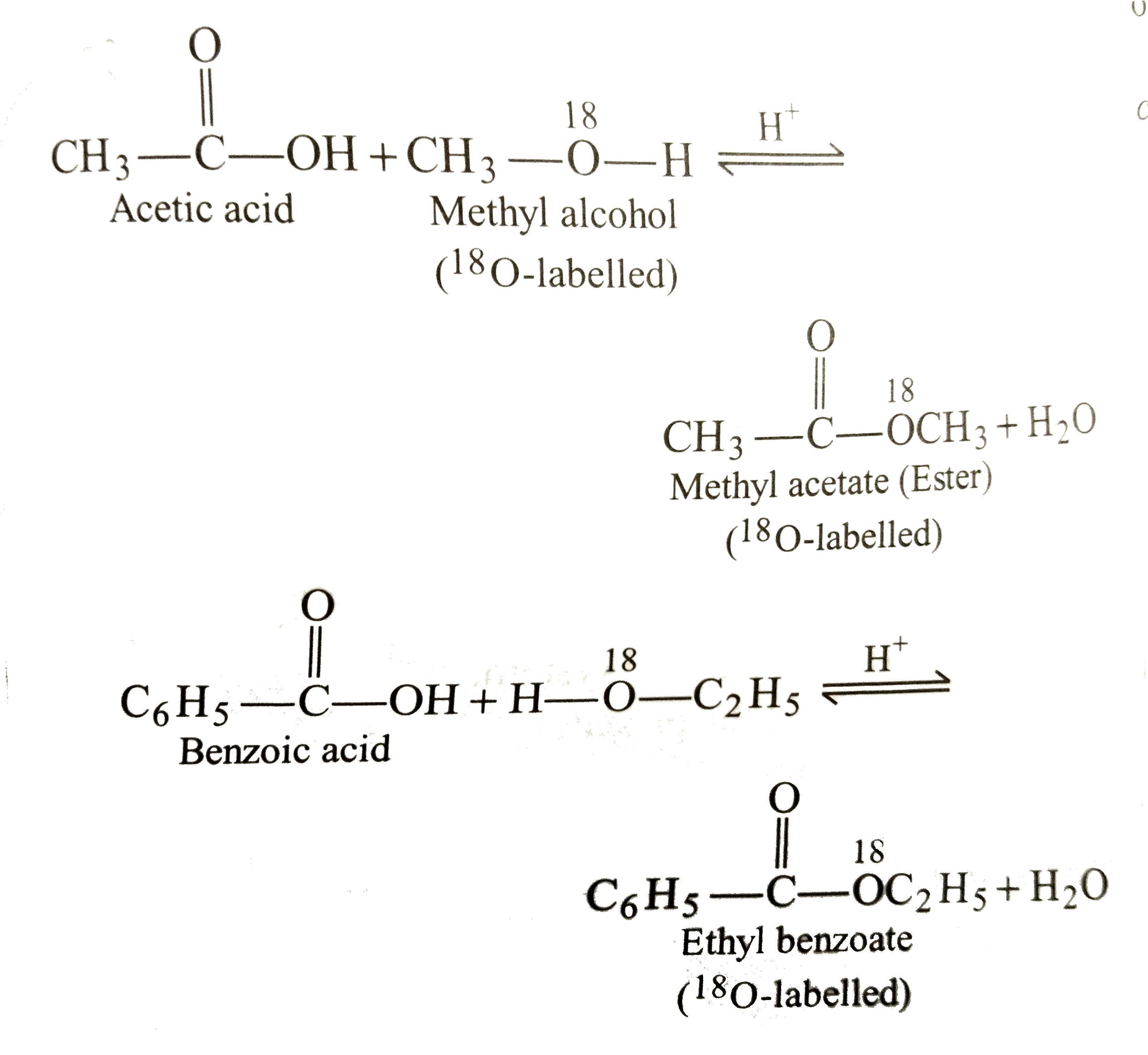

![PDF] Esterification of Acetic Acid with Ethanol Catalysed by an PDF] Esterification of Acetic Acid with Ethanol Catalysed by an](https://cdn.masterorganicchemistry.com/wp-content/uploads/2019/12/0-malonic-ester-synthesis-example-and-acetoacetic-ester-synthesis-example.gif)