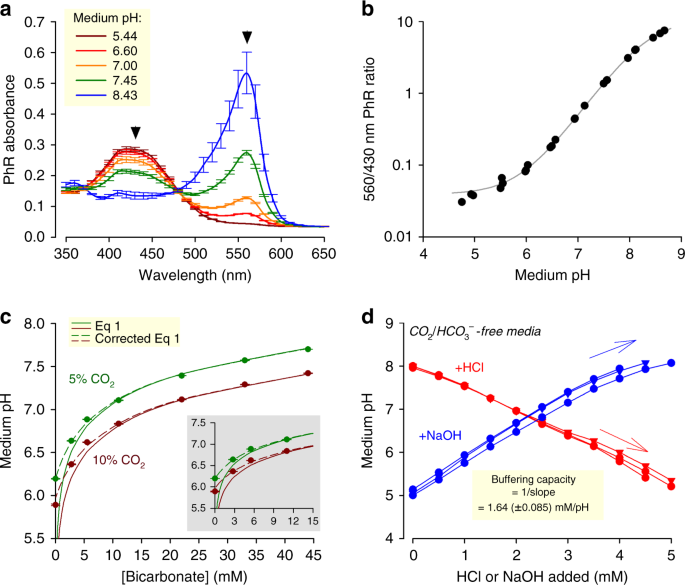
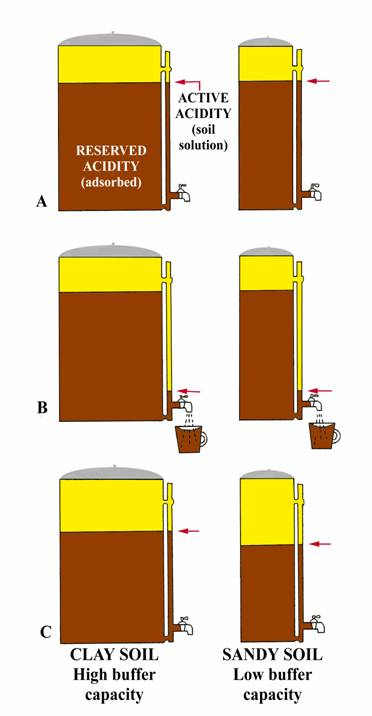
determining buffer capacity experiment
|
7—Investigation of Buffer Systems
Buffer capacity is a quantitative measure of the resistance of a buffer solution to change in pH on addition of strong acid or base Normally it can be defined |
|
Buffer Capacity
In this experiment you will prepare several different buffer solutions and determine the buffer capacity for each Part of the data analysis will include a |
|
Experiment-11-Chem-1Bpdf
The buffer will be prepared by choosing the appropriate acid-base pair calculating the molar ratio of acid to base that will produce the assigned pH and then |
How do you choose a buffer for an experiment?
Most buffers work best at a pH within 1 unit of their pKa at 20°C.
If you think your experiment will lower the environmental pH, then select a buffer with a pKa that's just a little lower than your working pH.
Likewise, select a buffer with a pKa slightly higher if you expect your experiment to raise your working pH.What is the buffer capacity of the buffer solutions to be used in the experiment?
Buffer capacity is a quantitative measure of the resistance of a buffer solution to change in pH on addition of strong acid or base.
Normally it can be defined as the millimoles of strong acid or base required to change the pH of 1 liter of buffer by ±1.0 unit.Testing the Buffer (1/10 strength) - (a) Measure 5.00 mL of buffer prepared in the previous step into a beaker and add 45 mL of distilled water into the beaker - mix.
Pipet 10 mL of the resulting diluted buffer into a large test tube.
Measure the pH.
Add 0.10 mL of 0.10 M HCl and measure the pH again.
What is the method of determination of buffer?
To calculate the pH of a buffer solution when base is added, the Henderson-Hasselbalch equation, pH = pKa + log (acid/base), is used.
The mol of base is added to the buffer's base, and the base's mol is subtracted from the buffer's acid.
These new mols are used to find the pH.
|
Buffer Capacity
In this experiment you will prepare several different buffer solutions and determine the buffer capacity for each. Part of the data analysis will include a |
|
Buffer capacity: An undergraduate laboratory experiment
buffer capacity for a given buffer system |
|
7—Investigation of Buffer Systems
Objectives. • Reinforce concepts of buffer buffer range and buffer capacity. • Learn how to prepare acid-base buffers. • Learn how to calculate the pH of a |
|
Buffers in Context: Baby Wipes As a Buffer System
26 févr. 2019 experiment seeks to prompt students to think beyond the macroscopic view ... determining what factors influence buffering capacity.3 One of. |
|
Experiment 11 - Chem 1B
buffer solution is determined by the Ka of the acid and by the ratio of concentrations of buffer is said to have a high buffer capacity. |
|
The Determination of Buffering Capacity of Some Ruminants
ruminant's feedstuffs and their additivity to calculate ration buffering capacity as a tools for feed formulation. The first experiment was performed with |
|
Calculation of the Buffering Capacity of Bicarbonate in the Rumen
04 mol/L final volume) and equilibrium pH was recorded when it was clearly stable |
|
Experiment # 9: The Henderson-Hasselbalch Equation
In general the buffering capacity is satisfactory over a pH range of pKa ± 1. A buffer solution of a given pH can be prepared by choosing a weak acid (or a |
|
DETERMINATION OF THE BUFFERING CAPACITY OF
In that study buffering capacities of several muscles from different species were determined by titrating with dilute acid or base. The roles of proteins |
|
Chapter 1.2: Visualization of Buffer Capacity with 3-D Topos: Buffer
14 déc. 2020 for teaching with BufCap TOPOS and derivation of new equations that permit the calculation of buffer capacities for titration/dilution ... |
|
7—Investigation of Buffer Systems - James Madison University
Reinforce concepts of buffer, buffer range and buffer capacity Learn how to calculate the pH of a buffer solution by using Henderson-Hasselbalch Equation Laboratory Notebook—prepared before lab (if require by your instructor) |
|
Buffer Capacity
Using the burettes, add the volumes of acetic acid and sodium acetate given in Table 1 to 100-mL flasks Then, fill the flasks to the fiducial mark with DI water The total buffer concentration (measured as total [OAc-] for these solutions is roughly 0 05M |
|
1 Experiment 10: Buffers Reading: Sections 161-162 in Olmstead
acid and the standardized NaOH solution that you used in the previous lab Finally A pH of Different Ratios of Weak Acid/Conjugate Base: Determine the Buffer Range A 0 100 M solution has sufficient buffering capacity for the exercise |
|
1 Experiment 6: Buffers Reading: Sections 161-162 in Olmstead
Experiment 6: Buffers Reading: A pH 5 or pH 9 buffer will be prepared using solid sodium acetate or For example, a 1:1 mixture of acetic acid (HOAc) and sodium A 0 100 M solution has sufficient buffering capacity for the exercise |
|
Experiment 32 BUFFERS1 - USNA
3 jan 2019 · The purpose of this experiment is to develop the concept and study the properties of buffers Calculate the change in pH of a simple buffer solution of known Capacity Useful Range Fill Factor Weak Acid/Base Buffer |
|
PH and Buffers Laboratory
buffering capacity In analyzing the pKa using the Henderson-Hasselbalch equation Buffers are the bottom of the electrode with a lab wipe The electrode |
|
CHEM 113 GENERAL CHEMISTRY LABORATORY-I BOOKLET
EXPERIMENT 7: BUFFERS, BUFFER CAPACITY, AND BUFFERING ZONE Determination of the reaction stoichiometry, calculation of the theoretical and |
|
Buffer Preparation and Capacity
In lab this week you are going to prepare an assigned buffer solution and test the buffering capacity of the solution using strong acid (HCl) and strong base (NaOH) determined using the Henderson-Hasselbalch Equation, and the closer the |
|
LEC 0616 Titration curves and buffering capacity - PHYWE System
Determine the buffering capacity of various aqueous acetic acid/sodium Prepare the solutions required for the experiment as follows: – 0 5 molar NaOH |
|
Lab 4: Designing and Preparing a Buffer - Bellevue College
Finally you will use equation (3) to design and prepare a buffer of a specific pH Procedure Part 1, pH of salt solutions: You will need a calibrated pH meter and 4 |