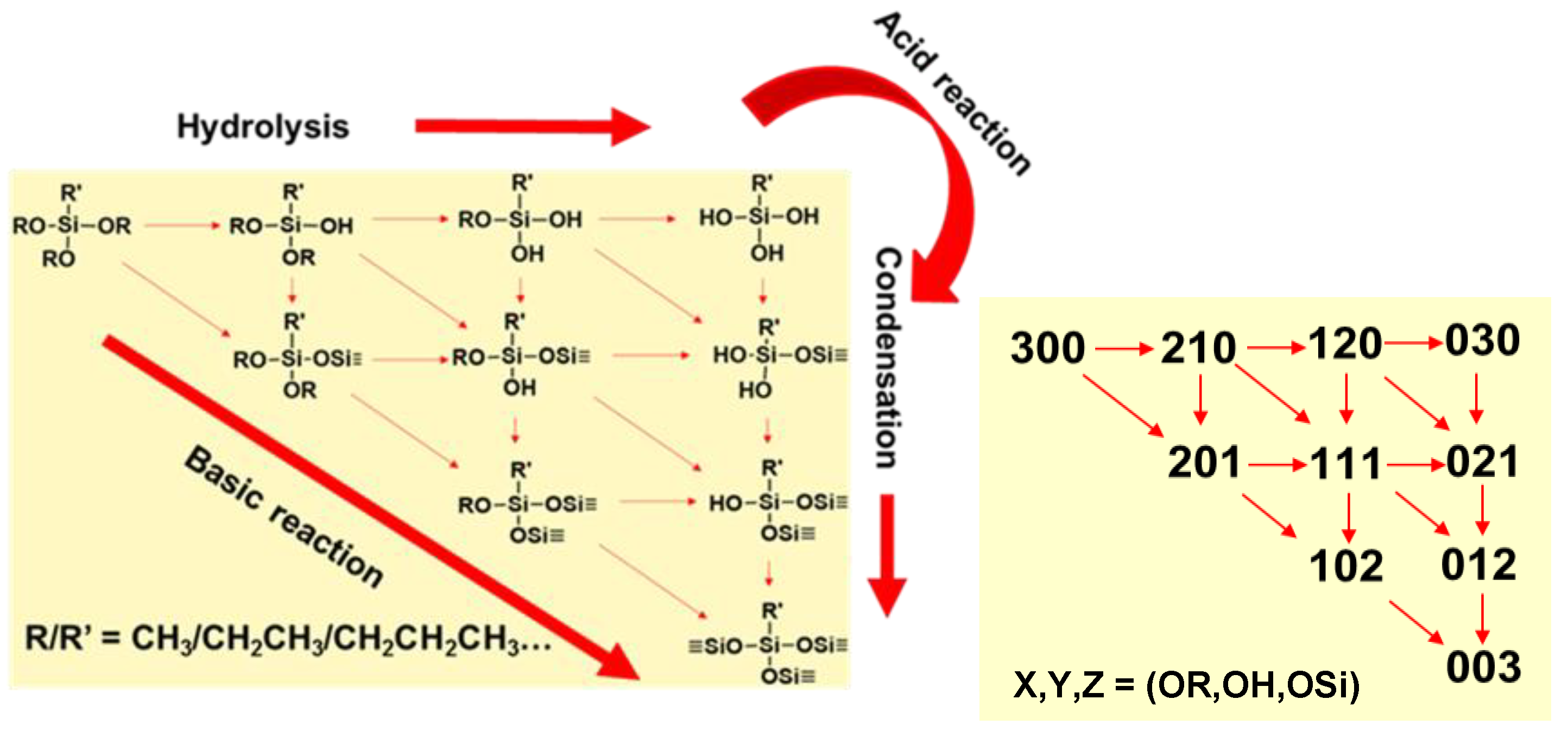
base catalyzed hydrolysis rna
This happens because the ribose sugar in RNA contains a hydroxyl (OH) group at the 2' position.
DNA does not have this feature and thus is not susceptible to hydrolysis, making it a more stable molecule.
What is the base hydrolysis of RNA?
The hydrolysis occurs at the phosphodiester bond of the backbone.
Hence, RNA rapidly hydrolyzes in an alkaline environment into cyclic 2',3'-monophosphate nucleotides, which undergo further hydrolysis in water to form a mixture of 2'- and 3'-nucleoside monophosphates.
What is the hydrolysis product of RNA?
RNA and DNA are nucleic acids which on complete hydrolysis yield a pentose sugar, phosphoric acid and nitrogen containing heterocyclic compounds called bases.
What is the purpose of using 1% NaOH in RNA hydrolysis?
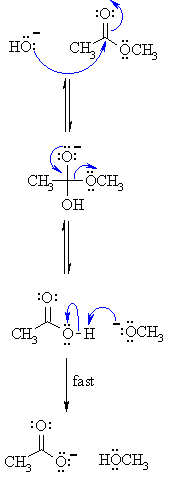
When RNA is treated with NaOH, the base catalyzes the cleavage of the phosphodiester bonds between the nucleotides, resulting in the release of individual nucleotides. 3.
Using a 1% NaOH solution provides a controlled environment for RNA hydrolysis.
|
Non-Enzymatic RNA Hydrolysis Promoted by the Combined
RNA RNA Degradation |
|
Mechanism of RNA Cleavage by Imidazole. Catalysis vs Medium
the imidazole-catalyzed hydrolysis of RNA and variousderiva- tives 1 (poly(U) UpU |
|
Properties of dianionic oxyphosphorane intermediates: implication
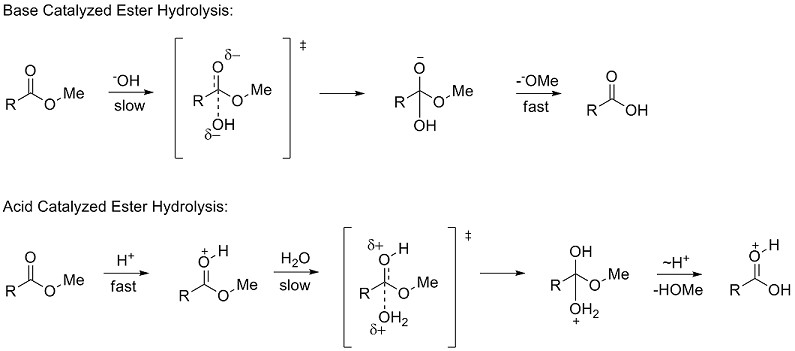
From calculations of a model reaction scheme for base-catalyzed RNA hydrolysis (which also represents the base-catalyzed methanolysis of ethylene phosphate |
|
Duplex Structure of Double-Stranded RNA Provides Stability against
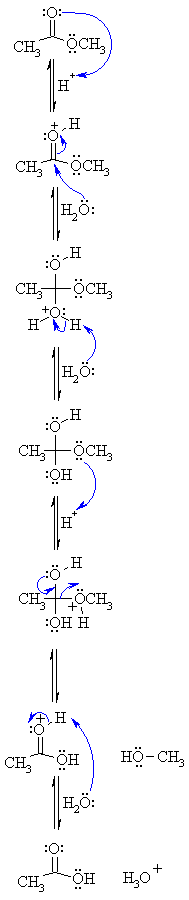
May 25 2021 Phosphodiester bonds undergo alkaline hydrolysis (also known as base-catalyzed hydrolysis or hydroxide-mediated hydrolysis) upon deproto- nation ... |
|
A general two-metal-ion mechanism for catalytic RNA
transition state ofthe reaction (iii) use of acid-base catalysis |
|
Sequential general base-acid catalysis in the hydrolysis of RNA by
The hydrolysis of RNA by bovine pancreatic ribonuclease involves catalysis by an imidazole group acting as a general base and an imidazolium group acting as |
|
Existence of a marginally stable intermediate during the base
base-catalyzed RNA hydrolysis5 a pentacoordinate oxy- phosphorane intermediate (2a) does exist at the 3-21G* level of theory. Moreover |
|
Trigger loop of RNA polymerase is a positional not acid–base
transcription |
|
Kinetics of RNA Degradation by Specific Base Catalysis of
Degradative mechanisms that spontaneously act upon DNA such as phosphoester hydrolysis |
|
Hydrolytic Glycosidic Bond Cleavage in RNA Nucleosides: Effects of
Nov 23 2016 U) |
|
Principles of Nucleic Acid Cleavage by Metal Ions - Squarespace
RNA cleavage catalyzed by alkali and metal-hydroxides is well known Both RNA and DNA can be cleaved by acid hydrolysis albeit by different mechanisms Surprisingly, the chemical stability of RNA at pH 5-6 may be even higher than that of DNA because DNA can be depurinated and cleaved at pH 8 (Ugarov et al |
|
Enormously Fast RNA Hydrolysis by Lanthanide(III) - The Journal of
Non-enzymatic hydrolysis of RNA is the subject of growing RNA (12, 22, 26, 28, 29), DNA (31-36), the catalysis and the base catalysis take place concertedly, |
|
Understanding the transition states of phosphodiester bond cleavage
and hydrolysis are catalyzed by numerous essential enzymes Two prominent mechanisms have been proposed for RNA and protein enzyme catalyzed cleavage of phosphodiester enzymes often invoke acid–base catalysis, active site |
|
Trigger loop of RNA polymerase is a positional, not acid - PNAS
7 jui 2017 · transcription proofreading acid–base catalysis trigger loop RNA hydrolysis Transcription of DNA-encoded messages into RNA is a |
|
Ab initio molecular orbital calculations on the base-catalyzed
In order to examine the energetics In base-catalyzed hydrolysis of RNA, a tentative pentacoordinated intermediate (3) has been characterized by molecular orbital |
|
Active site constraints in the hydrolysis reaction catalyzed by
and Steitz (4) for RNA-catalyzed hydrolysis and phosphoryl transfer reactions base pair is correlated with the propensity of E coli RNase P RNA to cleave at |
|
RNA Hydrolysis via an Oxyphosphorane - ScienceDirect
From calculations of a model reaction scheme for base-catalyzed RNA hydrolysis , a pentacoodinate dianionic intermediate a (Storer, et al , J Am Chem Sot , |















![acid base catalysed Ester hydrolysis - [PDF Document] acid base catalysed Ester hydrolysis - [PDF Document]](https://upload.wikimedia.org/wikipedia/commons/thumb/9/97/RNA_Hydrolysis.png/440px-RNA_Hydrolysis.png)