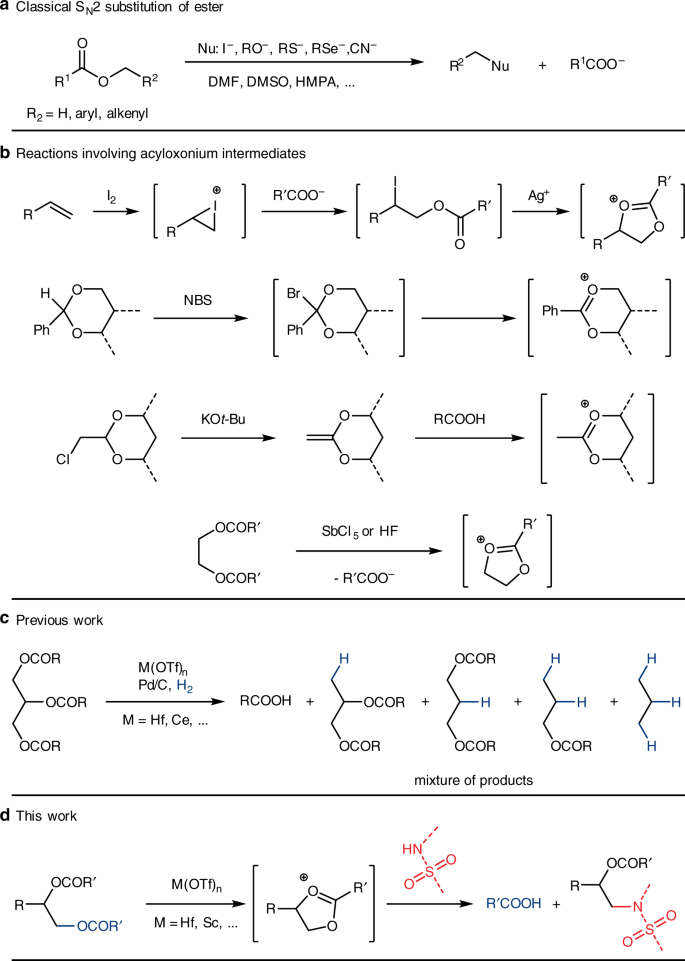
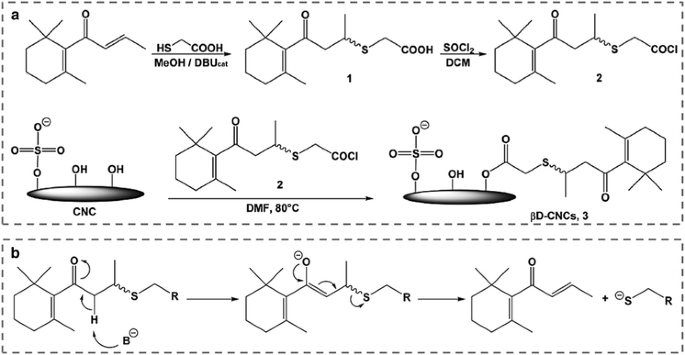
esterification reaction de substitution
Why is reaction 4 called esterification?
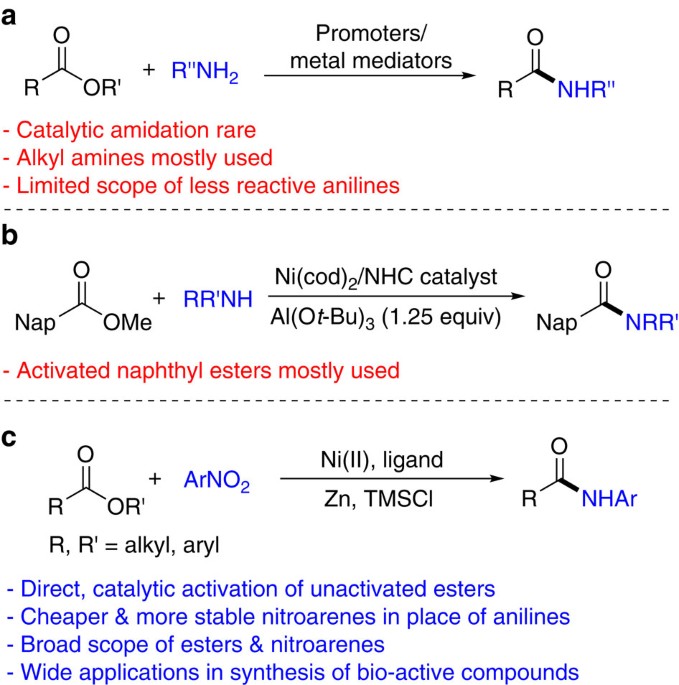
Reaction #4 is called esterification, since it is commonly used to convert carboxylic acids to their ester derivatives. Esters may be prepared in many different ways; indeed, equations #1 and #4 in the previous diagram illustrate the formation of tert-butyl and methyl esters respectively.
What is a transesterification reaction?
Transesterification is a reaction where an ester is converted to a different ester through reaction with an alcohol. Because there is typically very little difference in stability between both esters, the equilibrium constant of this reaction is usually near one.
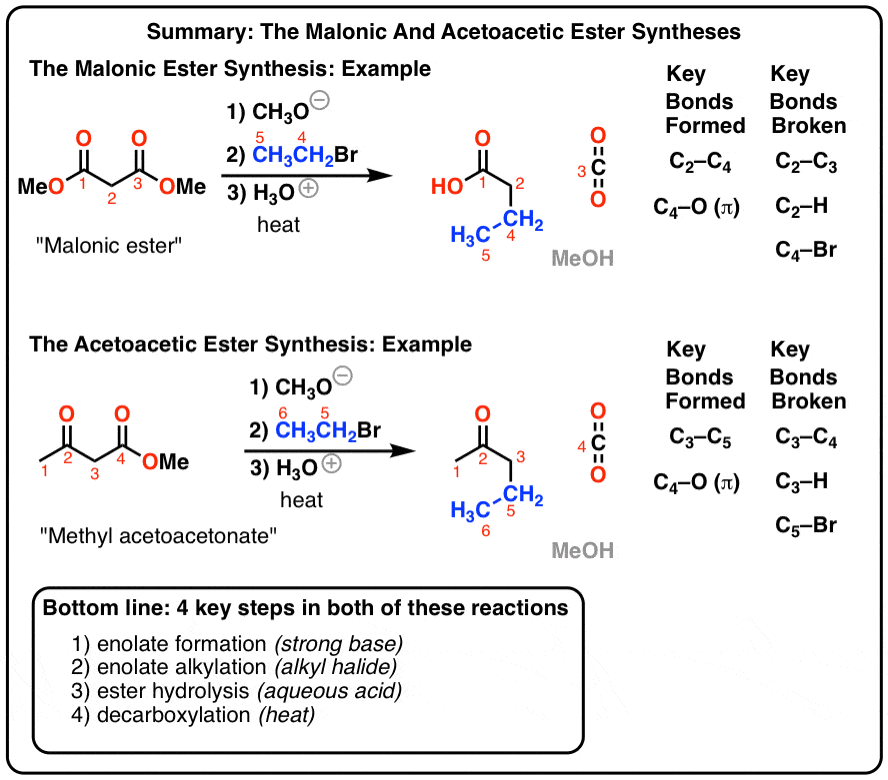
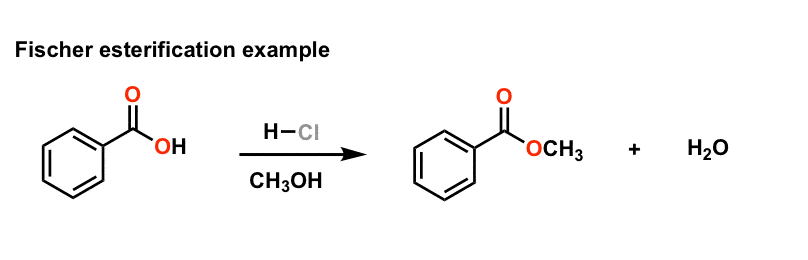
Conversion of Carboxylic Acids to Esters
Carboxylic acids can be converted to esterswith an acid catalyst and an excess of alcohol. This is known as Fischer esterification. or just “esterification of acids”. Common choices for acids include sulfuric acid (H2SO4), tosic acid (TsOH) and hydrochloric acid HCl among others. For our purposes these conditions can be considered to be equivalent
Intramolecular Fischer Esterification
The Fischer esterification can also be used to make cyclic esters (lactones). This is an example of an intramolecular reaction. The bonds that form and break are exactly the same But since the nucleophile and electrophile are attached to the same molecule, we obtain a cyclicproduct. Ring formation works best for the formation of 5- and 6-membered
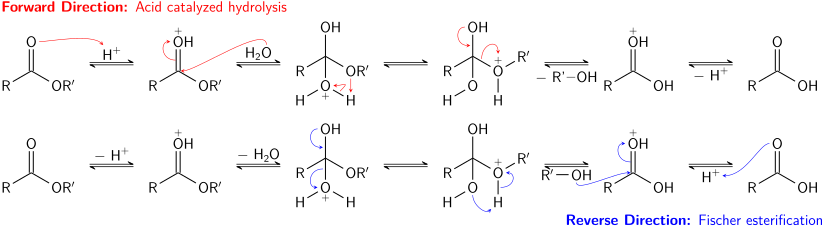
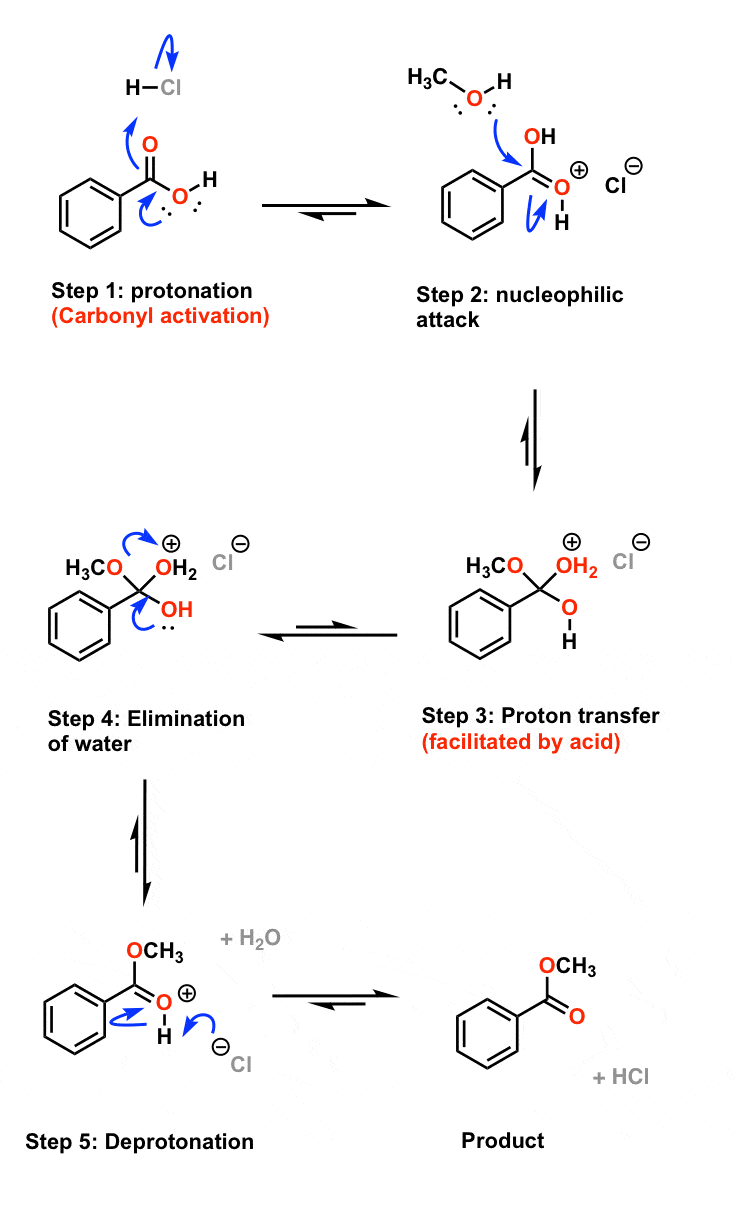
Mechanism of The Fischer Esterification
So how does this reaction work? The Fischer esterification mechanism has six steps. Each step is reversible and the starting materials and final product are all in equilibrium. In the first step of the Fischer esterification, the carbonyl oxygen is protonated with acid to give anoxonium ion. The resulting protonated carbonyl is an even better elect
Comparing Fischer Esterification with Saponification
Why does esterification of acids work well under acidic conditions, but fails under basic conditions? Recall that a key driving force for nucleophilic acyl substitution reactions is that they are strongly favorable when the nucleophile is a stronger base than the leaving group. [See post: Nucleophilic Acyl Substitution] When we employ acidic condit
What Is The Driving Force?
There’s one last question worth asking. What’s the actual driving force for the Fischer esterification? Usually, nucleophilic acyl substitution reactions follow the Principle of Acid-Base Mediocrity. That means that reaction proceeds in the direction in which the stronger base (nucleophile) displaces a weaker base (leaving group). [See post: Nucleo
Large Excess of Reagent
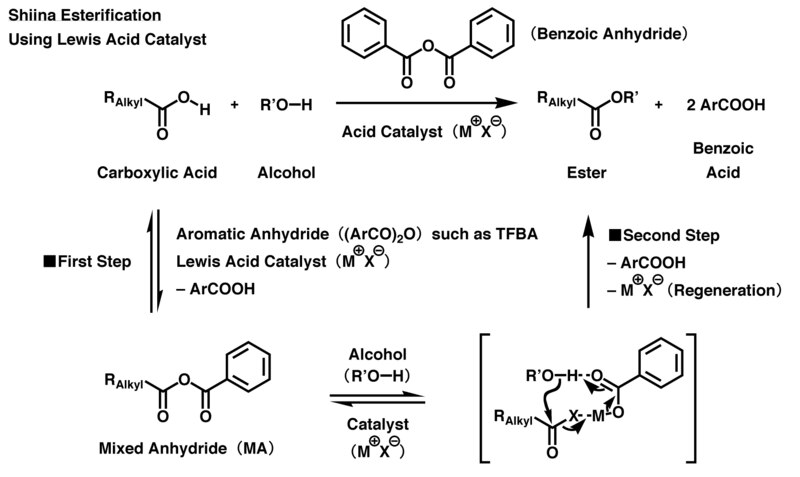
One study on this reaction [Note 3] used acetic acid and ethanol in the presence of an acid catalyst. It was found that running the Fischer esterification using equal amounts of acetic acid and ethanol gave a 65% yield of the ester at equilibrium. Not bad. But the reaction could be driven even further to the right by using a 10-fold excess of the a
Removing Water with A Dean-Stark Trap
Another way to ensure the reaction runs in the direction of ester formation is to removethe water as it is formed. This pushes the equilibrium towards the right via Le Chateliers’ principle. A clever way to do it is to use an apparatus called a Dean-Stark trap. In this process, a solvent such as benzene or toluene is used. These molecules co-distil
Summary
The Fischer esterification is one of those classic reactions in organic chemistry that is never going away. It is one of the cheapest and most effective processes for the formation of esters from carboxylic acids, especially on large scale. All of the steps in the Fischer esterification are potentially reversible. The six steps in the mechanism (Pr
Notes
Note 1. The equilibrium for the formation for unstrained, five- and six-membered rings is highly favorable for entropic reasons. A molecule of the leaving group (e.g. H2O) is released into solution with attendant increase in translational entropy (three degrees of translational entropy and up to three degrees of rotational entropy). This is sometim
References and Further Reading
References 1. First example Emil Fischer, Arthur Speier (1895). “Darstellung der Ester”. Chemische Berichte. 28: 3252–3258DOI: 1002/cber.189502803176Original paper by Emil Fischer and Arthur Speier describing acid-catalyzed esterification of carboxylic acids and alcohols. 2. Protonic States and the Mechanism of Acid‐Catalysed Esterification Dr. H.

Fischer Esterification Reaction Mechanism

Esterification reaction FSc. lecture organic chemistry mechanism of esterification

20.8 Synthesis and Reactions of Esters Organic Chemistry
|
LESTERIFICATION … correction
Il a été formé lors de la réaction entre l'acide éthanoïque et le butan-1-ol. 1.2. Il s'agit d'une réaction de substitution car le OH du groupe carboxyle de |
|
26 1. Lestérification est une substitution car de leau est formée en
2. La vitesse de réaction est augmentée en chauffant le milieu réactionnel et en ajoutant dans ce milieu de l'acide sulfurique un catalyseur. |
|
1 Les esters sont des composés organiques souvent à lorigine de l
par réaction entre un acide carboxylique R – COOH et un alcool R ' – OH. Indiquer si la réaction d'estérification est une réaction de substitution ... |
|
La substitution des solvants par les esters méthyliques dacides gras
Les esters d'acide gras d'huiles végétales sont obtenus à partir de la réaction entre un alcool et les acides gras provenant des huiles végétales. On parle des |
|
Synthèse dun ester par substitution nucléophile
l'iodure d'éthyle permet d'illustrer les réactions de substitution nucléophile au pro- la VSEPR de commenter les aspects stériques de l'estérification. |
|
Acides carboxyliques
3 oct. 2018 Réaction de substitution nucléophile sur carbone sp2 : ... Nous sommes dans le même cas que la réaction d'estérification des alcools. |
|
Substitution nucléophile et ?-élimination
ETUDES DES REACTIONS DE SUBSTITUTION NUCLEOPHILE . Lors d'une réaction de substitution un atome ou un groupe d'atomes remplace un atome. |
|
Chapitre 8-Les réactions de substitution
8.1.23c – Réactions avec les anhydrides esters carboxyliques |
|
Chimie organique
Dans ce cas-là la réaction d'élimination est souvent en compétition avec la formation d'étheroxydes par une réaction de substitution nucléophile. |
|
GRENOBLE 1 THESE DOCTEUR DE LUNIVERSITE JOSEPH
Détermination du degré de substitution par gravimétrie ............................. - 97 - ... La réaction d'estérification que nous avons mise en œuvre. |
|
LESTERIFICATION correction
Il a été formé lors de la réaction entre l'acide éthanoïque et le butan-1-ol 1 2 Il s' agit d'une réaction de substitution car le OH du groupe carboxyle de l'acide |
|
Facteurs favorisant une réaction de substitution nucléophile - UNF3S
Dans le cas des C=O des dérivés d'acide (acide carboxylique, ester, chlorure d' acide ), après addition du nucléophile, le C du C=O est lié à 3 groupements |
|
Réactions daddition-élimination - La physique-chimie et le cinéma
à un processus de substitution sur l'atome de carbone du carboxyle : Les réactions d'estérification et d'hydrolyse des esters sont parmi les plus étudiées de la |
|
Chimie organique - Chimie en PCSI
Afin que les alcools puisent donner lieu à des réactions de substitution et d' élimination, il faut tout d'abord que le groupe OH puisse être transformé en un bon |
|
Substitution nucléophile et β-élimination - Chimie en PCSI
ETUDES DES REACTIONS DE SUBSTITUTION NUCLEOPHILE Réponse : ester amide cétone alcool 2 Où ?? Question de régiosélectivité |
|
S6 Synthèse chimique
Cette réaction est chimio-sélective (N-acétylation plutô la nature de la famille de réaction mise en jeu (acide-base, oxydo-réduction, addition, substitution, C O OR' Ester Non stéréospécifique nucléophile δ+ site électrophile δ+ δ+ δ+ δ+ |
|
Formation des alcanes
4) Réactions de substitution nucléophile à partir des alcools R OH R X c) dérivés d'acides carboxyliques (halogénures d'acide, esters et anhydrides) : R |
|
COR 301 Chimie Organique II La Chimie du Carbonyle et des
2 2 3 Addition des carboxylates: préparation d'esters Les réactions de substitution nucléophile sur des carbones hybridés sp3 sont parmi les plus connues |
|
Substitution de solvants et matières actives de synthèse par - CORE
Puis, nous décrirons la réaction d'estérification des acides gras à chaîne courte et impaire Enfin, dans le troisième et dernier Chapitre nous nous consacrerons |